Jørgensen’s Organocatalysts
Introduction
Professor Karl Anker Jørgensen and his group have developed (R)- and (S)-α,α-bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether which serve as excellent chiral organocatalysts in the direct asymmetric α-functionalization of aldehydes.
In the field of asymmetric synthesis this stereoselective functionalization certainly represents an important breakthrough. Jørgensen’s diarylprolinol silyl ether reagents were shown to catalyze a variety of bond-forming reactions such as C–C, C–N, C–O, C–S and C–Hal in high yields with excellent levels of enantiocontrol.

Figure 1.pyrrolidinemethanol trimethylsilyl
Representative Applications
α-Amination
Direct α-amination between aldehydes and azodicarboxylates at room temperature using Jørgensen’s organocatalyst was reported to match enantioselectivities achieved by using l-proline as a catalyst. Interestingly, the aminated products were of inverted absolute configuration even though the absolute configuration of the catalyst is the same, due to steric shielding.

Figure 2.α-Amination
Domino Conjugated Nucleophilic Addition-Electrophilic Amination
A simple approach to highly functionalized molecules is Jørgensen’s one-pot multicomponent domino conjugated nucleophilic addition-electrophilic amination protocol. This procedure gives access to 1,2-aminothiol derivatives with enantioselectivities >99% ee. The soft sulfur nucleophile first reacts with the iminium ion intermediate formed by the α,β-unsaturated aldehyde, followed by the addition of the electrophile (i.e. the azodicarboxylate) to the enamine intermediate, yielding nearly enantiopure products when 2-[bis(3,5-bistrifluoromethylphenyl)trimethylsilanyloxy- methyl]pyrrolidine was used as the catalyst.

Figure 3.Domino Conjugated Nucleophilic Addition
Asymmetric Epoxidation
In addition to direct α-functionalization, Jørgensen also reported the first organocatalytic asymmetric epoxidation of α,β-unsaturated aldehydes using his sterically encumbered chiral pyrrolidine derivative under environmentally friendly reaction conditions (e.g. hydrogen peroxide as the oxidant). A series of differently substituted enals were transformed into the corresponding α,β-epoxy aldehydes with up to >90%, diastereoselectivities up to 98:2 and enantioselectivities up to 98%.
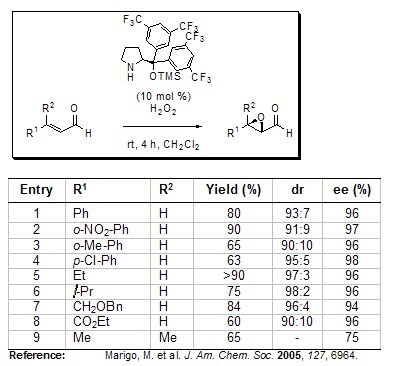
Figure 4.Asymmetric Epoxidation
Asymmetric Hydrophosphinylation
Very recently, Melchiorre and Córdova have simultaneously developed the first organocatalytic asymmetric hydrophosphinylation of α,β-unsaturated aldehydes, affording direct access to highly enantioenriched β-phosphine aldehydes. Advantageously, and in contrast to a metal-catalyzed process, the organocatalytic variant does not suffer from product inhibition arising from the coordination ability of the phosphorus atom. The chiral phosphines can provide, after simple manipulations, valuable bidentate P-ligands for metal-catalyzed enantioselective transformations.

Figure 5.Asymmetric Hydrophosphinylation
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?