Cell Counting Using a Hemocytometer

Aim
For the majority of manipulations using cell lines, such as transfections, cell fusion techniques, cryopreservation and subculture routines it is necessary to quantify the number of cells prior to use. Using a consistent number of cells will maintain optimum growth and also help to standardize procedures using cell cultures. This in turn gives results with better reproducibility.
Materials
- Cell Culture Media: pre-warmed to the appropriate temperature (refer to the ECACC cell line data sheet for the correct medium and temperature.)
- 70% (v/v) alcohol in sterile water (793213)
- Trypan Blue Solution (T8154)
- 0.05% trypsin/EDTA in HBSS, without Ca2+/Mg2+ (T3924)
Equipment
- Personal protective equipment (sterile gloves, laboratory coat, safety visor)
- Waterbath set to appropriate temperature
- Microbiological safety cabinet at appropriate containment level
- Centrifuge
- CO2 incubator
- Hemocytometer
- Inverted phase contrast microscope
- Pre-labelled flasks
Procedure
- Bring adherent and semi-adherent cells into suspension using trypsin/EDTA as described previously and resuspend in a volume of fresh medium at least equivalent to the volume of trypsin. For cells that grow in clumps centrifuge and resuspend in a small volume and gently pipette to break up clumps.
- Under sterile conditions remove 100-200 μL of cell suspension.
- Add an equal volume of Trypan Blue (dilution factor =2) and mix by gentle pipetting.
- Clean the hemocytometer.
- Moisten the coverslip with water or exhaled breath. Slide the coverslip over the chamber back and forth using slight pressure until Newton’s refraction rings appear (Newton’s refraction rings are seen as rainbow-like rings under the coverslip).
- Fill both sides of the chamber with cell suspension (approximately 5-10 μL) and view under an inverted phase contrast microscope using x20 magnification.
- Count the number of viable (seen as bright cells) and non-viable cells (stained blue). Ideally >100 cells should be counted in order to increase the accuracy of the cell count (see notes below). Note the number of squares counted to obtain your count of >100.
- Calculate the concentration of viable and non-viable cells and the percentage of viable cells using the equations below.
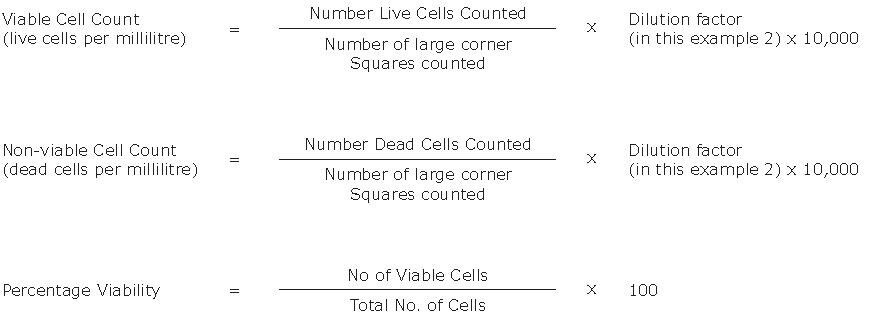
Key Points
1. Trypan Blue is toxic and is a potential carcinogen. Protective clothing, gloves and face/eye protection should be worn. Do not breathe the vapor.
2. The central area of the counting chamber is 1 mm2. This area is subdivided into 25 smaller squares (1/25 mm2). Each of these is surrounded by triple lines and is then further divided into 16 (1/400 mm2). The depth of the chamber is 0.1 mm.
3. The correction factor of 104 converts 0.1 mm3 to 1 mL (0.1 mm3 = 1 mm2 x 0.1 mm)
4. There are several sources of inaccuracy:
- The presence of air bubbles and debris in the chamber
- Overfilling the chamber such that sample runs into the channels or the other chamber
- Incomplete filling of the chamber Cells not evenly distributed throughout the chamber
- Too few cells to count. This can be overcome by centrifuging the cells, re-suspending in a smaller volume and recounting
- Too many cells to count. This can be overcome by using a higher dilution factor in trypan blue e.g. 1:10
5. The use of a hemocytometer can be time consuming, susceptible to subjective judgements by the operator and some cell types, such as those that form clusters, are particularly difficult to count using this method. Cell counting equipment is available offering alternative cell quantification methods including the Scepter™ Cell Counter. The Muse® Cell Analyzer enables flow cytometry-based assessment of cell count and viability. The Scepter™ Cell Counter is a portable, handheld cell counter that measures volume using the Coulter Principle. It can quantify cells based on size and will discriminate larger cells from smaller debris, unlike vision-based techniques, which rely on object recognition software and cannot reliably detect small cells. The Scepter™ cell counter detects every cell and displays the population as a histogram of cell size distributions. From the histogram, count all the cells or use the gating function to count a chosen subpopulation. By monitoring changes in your histogram, you can gain insight into the health and quality of your cell culture from one experiment to the next.

Figure 1.Counting cells using a hemocytometer and trypan blue. Viable cells contain intact cell membranes and do not uptake trypan blue, appearing bright/clear in the hemocytometer. Dead cells have damaged cell membranes and uptake trypan blue, appearing blue in the hemocytometer. Cell viability can be estimated by taking the ratio of live/dead cells.
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?