FFPE Tissue
Archived Formalin-fixed, Paraffin-embedded (FFPE) tissue samples are invaluable resources for profiling gene expression and studying a variety of diseases. Since the archived DNA is usually available in limited quantities, amplification of the samples is essential. Amplifying the FFPE tissue can be a difficult task due to the damaged template that results from the archiving process. This protocol provides a convenient method to purify and amplify genomic DNA from FFPE tissue. GenomePlex® Whole Genome Amplification has been used to amplify genomic DNA from rat and human FFPE tissue samples.
Note: The protocol below describes the process of DNA purification and subsequent whole genome amplification. We have dramatically streamlined this process with our GenomePlex® Tissue Whole Genome Amplification Kit (Product No. WGA2+C4482). This kit includes optimized reagents for tissue disruption and cell lysis eliminating the need for tedious organic extractions to remove excess paraffin or DNA purification prior to amplification.
Required Products
Materials to Be Supplied by the User
- FFPE tissue
- 37 °C water bath or heat block
- 55 °C water bath or heat block
- 70 °C water bath or heat block
- Xylene
- Ethanol (E7023)
- Water, molecular biology reagent (W4502)
- Microcentrifuge (with rotor for 2 mL tubes)
Extraction of DNA from Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue
This protocol was performed on rat liver tissue. The GenElute™ Mammalian Genomic DNA Miniprep Kit (G1N10) is recommended for this procedure.
- Place a small section (20 mg) of paraffin-embedded tissue in a 2 mL microcentrifuge tube.
- Add 1200 µL of xylene and vortex for 30 seconds.
- Centrifuge at full speed for 5 minutes at room temperature.
- Remove supernatant by pipetting. Do not remove any of the pellet.
- Add 1200 µL of ethanol to the pellet to remove the residual xylene. Mix by vortexing.
- Centrifuge at full speed for 5 minutes at room temperature.
- Carefully remove the ethanol by pipetting. Do not remove any of the pellet.
- Repeat steps 5–7 again.
- Incubate the open microcentrifuge tube at 37 °C for 10–15 minutes to remove any residual ethanol by evaporation.
- Digest Tissue: Resuspend the tissue pellet in 180 µL of Lysis Solution T.
- Add 20 µL of Proteinase K. Mix by vortexing. Incubate at 55 °C overnight or until the tissue is completely lysed. Vortex occasionally during incubation.
Optional RNase treatment: If residual RNA is a concern, add 20 µL of RNase A solution and incubate at room temperature for 2 minutes. - Lyse cells: Vortex for 15 seconds. Add 200 µL of Lysis Solution C to the sample. Vortex thoroughly as a homogenous mixture is essential for efficient lysis. Incubate at 70 °C for 10 minutes.
- Prepare column: Add 500 µL of the Column Preparation Solution to each pre-assembled GenElute Miniprep Binding Column and centrifuge at 12,000 × g for 1 minute.
- Prepare for binding: Add 200 µL of ethanol to the lysed sample and mix by vortexing.
- Load lysate: Transfer the entire contents of the sample tube into the treated binding column from step 14. Centrifuge at ≥6500 × g for 1 minute. Discard the collection tube containing the flow-through liquid and place the binding column in a new 2 mL collection tube.
- First wash: Prior to first use, dilute the Wash Solution Concentrate with ethanol as described under preparation instructions. Add 500 µL of Wash Solution to the binding column and centrifuge for 1 minute at ≥6,500 × g. Discard the collection tube containing flow-through liquid and place the binding column in a new 2 mL collection tube.
- Second wash: Add another 500 µL of Wash Solution to the binding column and centrifuge for 3 minutes at maximum speed (12,000–18,000 × g) to dry the binding column. It is crucial that the binding column is free of ethanol before eluting DNA off the column. Centrifuge the column for an additional minute if residual ethanol is visible. Discard the collection tube containing the flow-through liquid and place the binding column in a new 2 mL collection tube.
- Elute DNA: Pipette 200 µL of the Elution Solution directly into the center of the binding column and incubate at room temperature for 5 minutes. Centrifuge for 1 minute at ≥6500 × g to elute the DNA.
- Store DNA samples at –20 °C.
Note: This protocol can be performed without using xylene starting with step number 10. As a result of omitting the xylene treatment step, the amount of DNA will decrease by approximately 50% when compared to the protocol with a xylene step.
Application Data
GenomePlex® Whole Genome Amplification FFPE Tissue
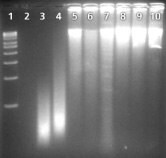
Legend: Products were amplified using the GenomePlex® Whole Genome Amplification Kit (WGA1) from us, Supplier A, and Supplier Q. Products were resolved on a 1.5% agarose gel. 5 µL of amplified product was added to each well. The products amplified using GenomePlex technology were of a smaller molecular weight as shown on the gel when compared to Supplier A and Q. This is due to the random fragmentation of genomic DNA prior to amplification. Our amplified products are specific and there is no amplicon visible in the negative control (lane 2) indicating that only the desired genomic DNA is amplified. Both Suppliers A and Q yield a nonspecific signal in the negative control which is equal in size and intensity to the signal for the suppliers’ positive control.
| Lane 1—1 kb Marker | Lane 6—Supplier A 10 ng input DNA |
| Lane 2—Our Negative Control | Lane 7—Supplier A 100 ng input DNA |
| Lane 3—Our 10 ng input DNA | Lane 8—Supplier Q Negative Control |
| Lane 4—Our 100 ng input DNA | Lane 9—Supplier Q 10 ng input DNA |
| Lane 5—Supplier A Negative Control | Lane 10—Supplier Q 100 ng input DNA |

Legend: DNA was extracted from a sample of formalin-fixed, paraffin-embedded rat liver. 10 ng and 100 ng of genomic DNA was amplified using GenomePlex® Complete WGA Kit (Product No. WGA2) followed by purification using the GenElute™ PCR Clean-up Kit (NA1020). Quantitative PCR (45 cycles) was performed on the WGA reaction using 12 different primer sets. GenomePlex demonstrates ~1000 fold (10 Ct) better representation compared to suppliers.
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?