USP Dissolution Testing Method (HPLC) for Folic Acid Tablets Using a Monolith Column and UV detection
Folic Acid Tablets (USP)

Folic acid or folate is classified as a B vitamin (B9).
Folic acid is synthetically produced, and used in fortified foods and supplements.
Folate is converted by humans to dihydrofolate (dihydrofolic acid), tetrahydrofolate (tetrahydrofolic acid), and other derivatives, which have various biological activities.
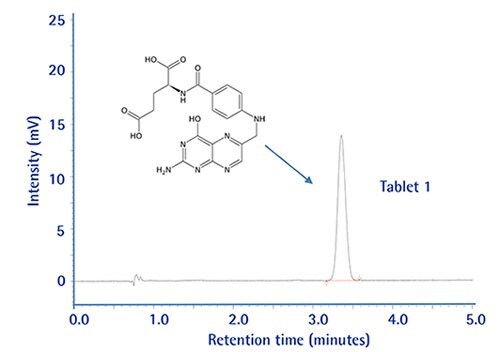
Drug dissolution testing has been carried out following the experimental conditions in the USP37-NF32 monograph for Folic Acid Tablets (using an isocratic HPLC method with RP-18 endcapped columns and thus scalable).
A 250x4.6 mm column is prescribed with L1 packing operating at 1.0 mL/min. To improve sample throughput we have transferred this method to a 100x4.6 mm long monolithic column.
The new method turned out to be faster, having improved chromatographic resolution, lower column backpressure, and still meeting all method performance criteria compared to the prescribed column.
Dissolution <711> HPLC
Test 1
Medium: Water; 500 mL
Apparatus 2: 50 rpm
Time: 45 min
Standard solution: Solution having a known concentration of USP Folic Acid RS, corrected for water content, in Medium
Sample solution: Filtered portion of the solution under test, suitably diluted with the Medium if necessary
Analysis
Samples: Standard solution and Sample solution
Proceed as directed in the Assay, making any necessary modifications.
Calculate the percentage of the labeled amount of folic acid (C19H19N7O6) dissolved:
Result = (rU/rS) × (CS×D×V/L) × 100
rU = peak area of folic acid from the Sample solution
rS = peak area of folic acid from the Standard solution
CS = concentration of USP Folic Acid RS in the Standard solution (mg/mL)
D = dilution factor for the Sample solution
V = volume of Medium, 500 mL
L = label claim (mg/Tablet)
Assay
Mobile phase: Transfer 35.1 g of sodium perchlorate and 1.40 g of monobasic potassium phosphate to a 1-L volumetric flask. Add 7.0 mL of 1 N potassium hydroxide and 40 mL of methanol, dilute with water to volume, and mix. Adjust with 1 N potassium hydroxide or phosphoric acid to a pH of 7.2.
Diluent: Aqueous solution containing 2 mL of ammonium hydroxide and 1 g of sodium perchlorate per 100 mL
System suitability solution: 0.2 mg/mL each of USP Folic Acid RS and USP Folic Acid Related Compound A
RS in Diluent. [Note—Before use, pass through a filter of 1-μm or finer pore size. ]
Standard solution: 0.20 mg/mL of USP Folic Acid RS, corrected for water content in Diluent
Sample solution: Equivalent to 0.2 mg/mL of folic acid, from NLT 20 powdered Tablets in Diluent; shake gently to aid dissolution, and filter, discarding the first portion.
Chromatographic system (see Chromatography 621, System Suitability.)
Detector: UV 254 nm
Column: 250x4.6 mm; packing L1
Flow rate: 1 mL/min
Injection volume: 25 μL
System suitability requirement
Resolution NLT 3.6 between folic acid related compound A and folic acid
Chromolith® HighResolution RP-18 endcapped

Chromatographic Data: Standard
Result (%) = (rU/rS) × (CSx D x V/L) ×V× 100
Tolerance: NLT 75% (Q) of the labeled amount of folic acid is dissolved.
Per continuare a leggere, autenticati o crea un account.
Non hai un Account?