D54702
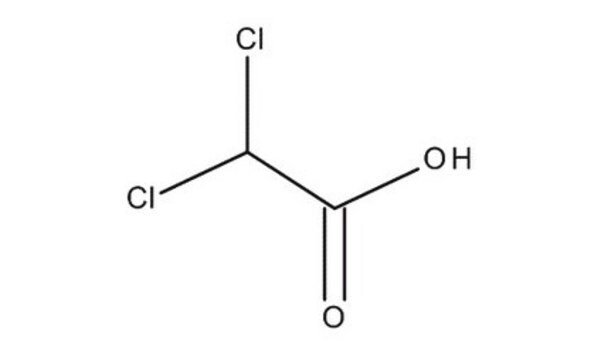
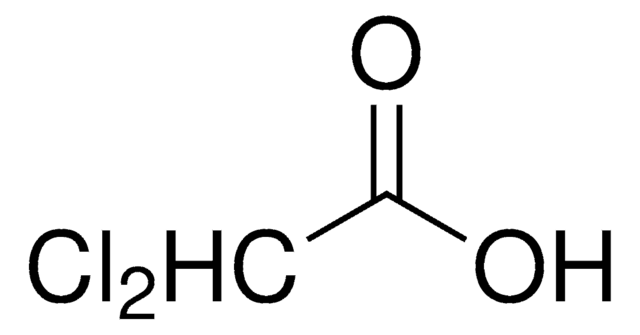
Dichloroacetic acid
ReagentPlus®, ≥99%
Sinonimo/i:
2,2-Dichloroacetic acid, DCA, DCAA
About This Item
Prodotti consigliati
Densità del vapore
4.5 (vs air)
Livello qualitativo
Tensione di vapore
0.19 mmHg ( 20 °C)
Nome Commerciale
ReagentPlus®
Saggio
≥99%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.466 (lit.)
P. eboll.
194 °C (lit.)
Punto di fusione
9-11 °C (lit.)
Densità
1.563 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
OC(=O)C(Cl)Cl
InChI
1S/C2H2Cl2O2/c3-1(4)2(5)6/h1H,(H,5,6)
JXTHNDFMNIQAHM-UHFFFAOYSA-N
Informazioni sul gene
human ... ALDH1A1(216) , PDK1(5163)
rat ... Pdk1(116551)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- A reactant in the synthesis of chloroketones by reacting with esters in the presence of LiHMDS via Claisen-type homologation reaction.
- A structure-directing agent, solvent, or plastdopant for the preparation of different morphologies of polyaniline (PANI). Self-assembling nanostructured PANI may be formed due to the presence of strong hydrogen bonding between DCA and aniline/polyaniline.
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Dermal - Aquatic Acute 1 - Carc. 2 - Eye Dam. 1 - Lact. - Met. Corr. 1 - Repr. 1B - Skin Corr. 1A - STOT RE 2 Oral
Organi bersaglio
Brain,Liver,Testes
Rischi supp
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.