365572
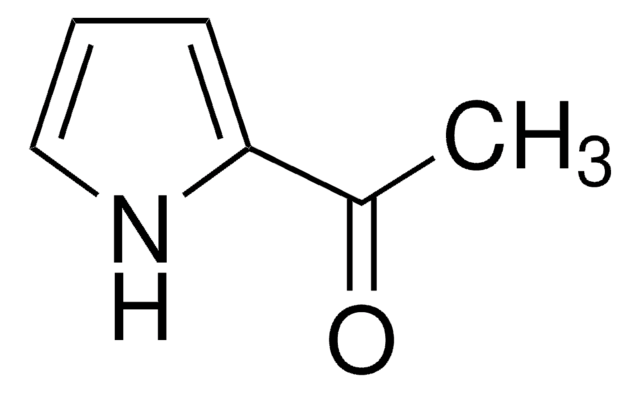
Methyl 6-methoxy-2-indolecarboxylate
99%
Sinonimo/i:
2-Methoxycarbonyl-6-methoxyindole, 6-Methoxyindole-2-carboxylic acid methyl ester
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H11NO3
Numero CAS:
Peso molecolare:
205.21
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Punto di fusione
117-119 °C (lit.)
Gruppo funzionale
ester
Stringa SMILE
COC(=O)c1cc2ccc(OC)cc2[nH]1
InChI
1S/C11H11NO3/c1-14-8-4-3-7-5-10(11(13)15-2)12-9(7)6-8/h3-6,12H,1-2H3
OPUUCOLVBDQWEY-UHFFFAOYSA-N
Applicazioni
Methyl 6-methoxy-2-indolecarboxylate is suitable for use in the production of dyes by Escherichia coli expressing naphthalene dioxygenase (NDO) and toluene dioxygenase (TDO). It is also suitable for use in the production of dyes by Escherichia coli expressing multicomponent phenol hydroxylase (mPH) from Pseudomonas sp. strains KL33 and KL28.
Reactant for preparation of:
- Benzoxazole containing indole analogs as peroxisome proliferator-activated receptor-γ/δ dual agonists
- Potent antiproliferative agent against human leukemia K562 cells
- Indole-indolone scaffold via [3+2] annulation of arynes
- Latonduine derivatives via intramolecular Heck reaction as possible anticancer agents
- Arylthioindoles as potent inhibitors of tubulin polymerization
- Heterocycle-fused derivatives of 1-oxo-1,2,3,4-tetrahydropyrazine via Ugi condensation
- Indole fatty alcohols (IFAs) as promoters of differentiation of neural stem cell derived neurospheres into neurons. Potential application for treatment of neurodegenerative diseases
- Light-dependent tumor necrosis factor-α antagonists
- 2-substituted indole melatonin receptor ligands
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
J Y Kim et al.
Letters in applied microbiology, 36(6), 343-348 (2003-05-20)
To isolate and characterize the phorate [O,O-diethyl-S-(ethylthio)methyl phosphoradiothioate] degrading bacteria from agricultural soil, and their assessment for multifarious biological activities of environmental and agronomic significance. Based on their morphological and biochemical characteristics, the selected isolates PS-1, PS-2 and PS-3 were
J Y Kim et al.
Letters in applied microbiology, 41(2), 163-168 (2005-07-22)
To establish multicomponent phenol hydroxylases (mPHs) as novel biocatalysts for producing dyestuffs and hydroxyindoles such as 7-hydroxyindole (7-HI) from indole and its derivatives. We have isolated Pseudomonas sp. KL33, which possesses a phenol degradation pathway similar to that found in
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.