Cell Migration & Invasion Assays
The Boyden Chamber Assay
Cell Migration Assays
Cell Invasion Assays
Microfluidic Migration Device
In Vitro Scratch Assay
ECM Proteins
References
Metastasis is the cumulative result of multiple changes in tumor cells and their microenvironment that enables cellular migration and invasion into healthy host tissue. As proliferating neoplastic cells attempt to escape the primary tumor site, local cell adhesion and invasion of the surrounding tissue must occur1. Prior to penetrating the blood vessel endothelium and gaining access to the blood stream, cancer cells must invade local tissues by degrading ECM protein components and ultimately, transverse the basement membrane2. Once in circulation, these cells can form metastatic colonies at secondary locations.

Figure 1.Tumor metastasis is a multistep process involving cancer cell adhesion, cell migration and invasion into neighboring tissues and vasculature.
The Boyden Chamber Assay
The most widely accepted cell migration technique is the Boyden Chamber assay3. The classic transwell migration assay system uses a hollow plastic chamber, sealed at one end with a porous membrane. This chamber is suspended over a larger well which may contain medium and/or chemoattractants. Cells are placed inside the Chamber and allowed to migrate through the pores, to the other side of the membrane. Migratory cells are then stained and counted.
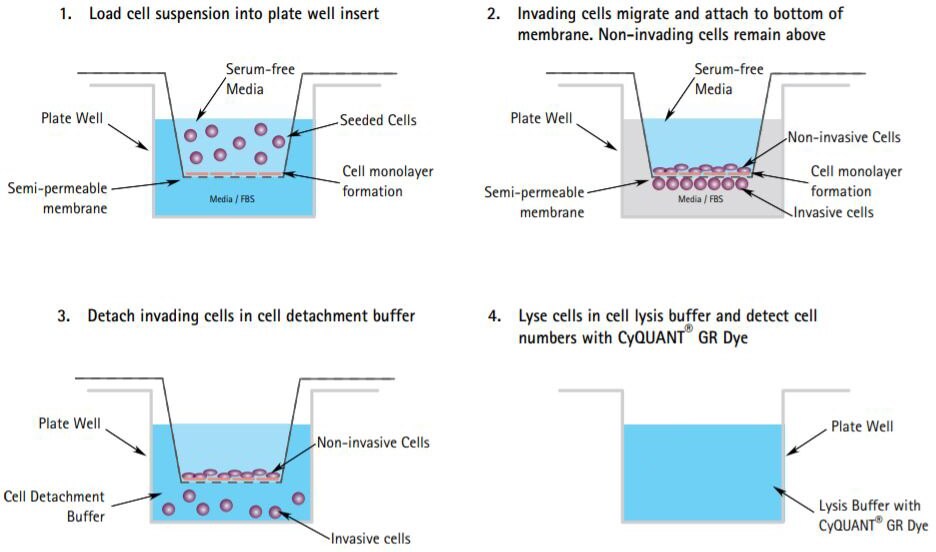
Figure 2.The Boyden Chamber Assay Protocol. Cells are allowed to migrate through a cell monolayer or ECM protein mixture which have been seeded onto a semi-permeable membrane cell culture insert with chemoattractants added below the membrane. Migrated cells can then be quantified by staining cells with DNA dyes such as Calcein-AM or CyQUANT GR Dyes
Cell Migration & Invasion Kits
Millipore’s QCM™ cell migration and invasion assays provide a quick and efficient system for the quantitative determination of various factors on cell migration, adhesion and invasion including screening of pharmacological agents, evaluation of integrins, chemoattractant or other adhesion receptors responsible for cell migration. The kits have provided researchers with consistent results for over a decade and have been published in over 4000 Publications.
Cell Migration Assays: Enables convenient and sensitive quantification of in vitro cell migration towards a chemical concentration gradient (chemotaxis) or ECM protein gradient (haptotaxis).
Cell Invasion Assays: Enables convenient and sensitive quantification of in vitro cell invasion through a basement membrane ECM protein or a layer of cells such as endothelial cells.
How to select the appropriate pore size for your cells:
- 3 μm pore size is appropriate for leukocyte or lymphocyte migration.
- 5 µm pore size is appropriate for a subset of fibroblast cells or cancer cells such as NIH-3T3 and MDA-MAB 231 cells. Also suitable for monocytes and macrophages.
- 8 μm pore size is appropriate for most cell types. This pore size supports optimal migration for most epithelial and fibroblast cells. Note - the 8 μm pore size is not appropriate for lymphocyte migration experiments.
Cell Migration Assays
Microfluidic Migration Device
Millicell® µ-Migration Assay Kit overcomes the limitations of traditional multiwell migration assays. The innovative design of the µ-Migration Slide promotes a stable diffusion-generated concentration gradient that is consistently linear and lasts for more than 48 hours. Made from a plastic with high optical qualities similar to those of glass, the µ-Migration Slide is specially designed for video microscopy assays. At specific time intervals, images of the observation area can be acquired, allowing real-time monitoring and quantitative measurements of cell migration.

Figure 3.Live Cell Migration of HT1080 cells. The effect of serum concentrations (0% or 10%) on the migration propensity of HT1080 cells on collagen-coated surface. The results show that the majority of the cells have directed migration towards 10% FCS gradient (Black).
In Vitro Scratch Assay
The scratch assay is a popular method for the study of cell migration4. Scaling up this technique has proved challenging, however, making biochemical analysis of the molecular events mediating wound repair difficult. The Cell Comb™ scratch assay addresses the need for a simple tool able to create multiple scratch wounds in a higher-throughput manner.


Figure 4.Using the CellComb scratch assay, monolayers of NIH3T3 cells were scratched/wounded in a one-direction (left) or two-direction (right) pattern. Over time, migrating cells will fill in the scratched sections and quantified using simple cell counting. Live cell imaging on migrating cells is also possible using the scratch migration assay.
ECM Proteins
Extracellular matrix (ECM) proteins are produced intracellularly and are subsequently secreted into the surrounding cellular medium, actively regulating a diverse range of cell functions including cell adhesion, differentiation, proliferation, migration, invasion and survival. A primary utility of ECMs in in vitro culture is to promote cellular adhesion while maintaining cell viability and maximizing cell proliferation for downstream cell-based applications.
- Collagen
- Laminin
- Fibronectin
- Vitronectin
- Elastin
- Basement Membrane Extract
References
To continue reading please sign in or create an account.
Don't Have An Account?