H21101
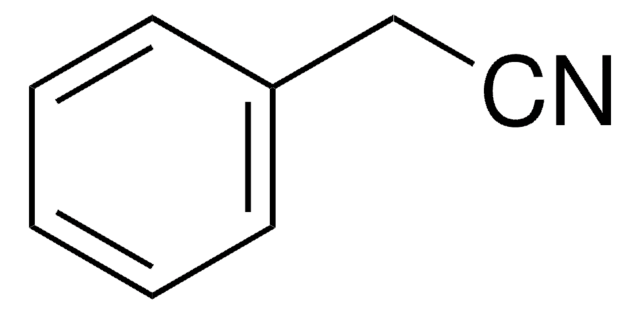
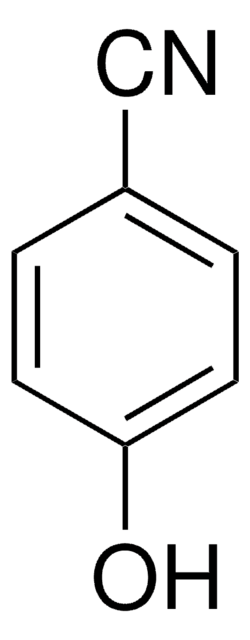
4-Hydroxyphenylacetonitrile
98%
Synonym(s):
4-Hydroxybenzyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4CH2CN
CAS Number:
Molecular Weight:
133.15
Beilstein:
1934470
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
330 °C/756 mmHg (lit.)
mp
67-70 °C (lit.)
SMILES string
Oc1ccc(CC#N)cc1
InChI
1S/C8H7NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5H2
InChI key
AYKYOOPFBCOXSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Buskov et al.
Journal of biochemical and biophysical methods, 43(1-3), 157-174 (2000-06-28)
In the present study analytical and preparative supercritical fluid chromatography (SFC) were used for investigation of myrosinase catalysed degradation of 4-hydroxybenzylglucosinolate (sinalbin). Sinalbin occurs as a major glucosinolate in seeds of Sinapis alba L., in various mustards and other food
L B Willis et al.
Canadian journal of microbiology, 44(6), 554-564 (1998-09-12)
Defined insertion mutations have been constructed in the Rhizobium (Sinorhizobium) meliloti phbC gene, which encodes poly-beta-hydroxybutyrate (PHB) synthase. The locus was isolated and subcloned from a genomic library of R. meliloti Rm1021 by complementation of phbC mutation of Alcaligenes eutrophus.
C J Cooksey et al.
The Biochemical journal, 333 ( Pt 3), 685-691 (1998-07-25)
When 3,4-dihydroxybenzylcyanide (DBC) is oxidized by mushroom tyrosinase, the first visible product, identified as the corresponding quinomethane, exhibits an absorption maximum at 480 nm. Pulse-radiolysis experiments, in which the o-quinone is formed by disproportionation of semiquinone radicals generated by single-electron
J M Baldoni et al.
The Journal of biological chemistry, 255(19), 8987-8990 (1980-10-10)
Dopamine beta-hydroxylase (EC 1.14.17.1) is inactivated by p-hydroxybenzylcyanide (PHBC) in a manner characteristic of a suicide substrate. The inactivation 1) is first order in inhibitor (Kd = 1.9 mM, k2 = 0.05 min-1, pH 5.0), 2) exhibits saturation kinetics, and
G Colombo et al.
The Journal of biological chemistry, 259(3), 1607-1615 (1984-02-10)
Dopamine beta-hydroxylase was incubated with p-hydroxybenzyl cyanide, ascorbate, and O2 and the products of the hydroxylation reaction were monitored by high performance liquid chromatography. At early times, p-hydroxymandelonitrile was the sole product but this compound slowly decomposed to p-hydroxybenzaldehyde and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![{6-[(2,2-Dimethylpropanoyl)amino]-3-pyridinyl}boronic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/362/081/7caa2220-e92f-4f6a-bec3-782fcb05df6e/640/7caa2220-e92f-4f6a-bec3-782fcb05df6e.png)