All Photos(1)
About This Item
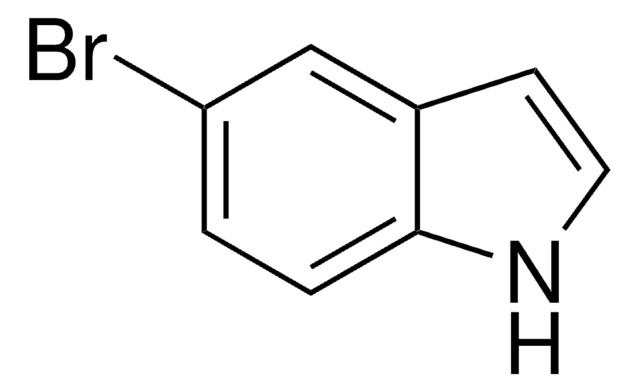
Empirical Formula (Hill Notation):
C8H6FN
CAS Number:
Molecular Weight:
135.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay
97%
bp
90 °C/0.4 mmHg (lit.)
mp
30-32 °C (lit.)
SMILES string
Fc1cccc2[nH]ccc12
InChI
1S/C8H6FN/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H
InChI key
ZWKIJOPJWWZLDI-UHFFFAOYSA-N
Application
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for preparation of antifungal agents
- Reactant for preparation of Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Reactant for preparation of Potent Selective Serotonin Reuptake Inhibitors
- Reactant for preparation of Inhibitors of HIV-1 attachment
- Reactant for preparation of monoamine reuptake inhibitors
- Reactant for preparation of histone deacetylase (HDAC) inhibitors
- Reactant for preparation of inhibitors of proliferation of human breast cancer cells
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
4-Fluoroindole and Derivatives.
Bentov M, et al.
Israel J. Chem., 2(1), 25-28 (1964)
A synthesis of (-)-indolactam V.
Semmelhack MF and Rhee H.
Tetrahedron Letters, 34(39), 1395-1398 (1993)
(Erratum)
Journal of Medicinal Chemistry, 2442-2442 null
Journal of the Chemical Society. Perkin Transactions 1, 1765-1765 (1994)
Synthesis of (L)-4-Fluorotryptophan.
Konas DW, et al.
Synthetic Communications, 42(1), 144-152 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service