M4414
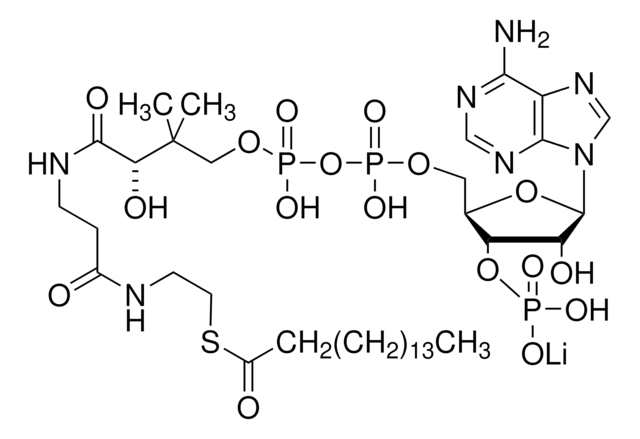
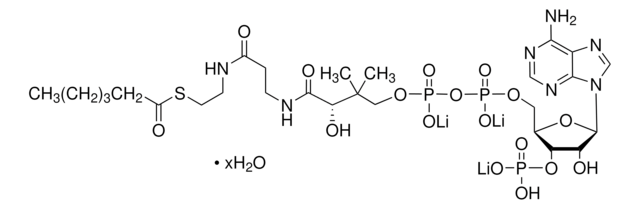
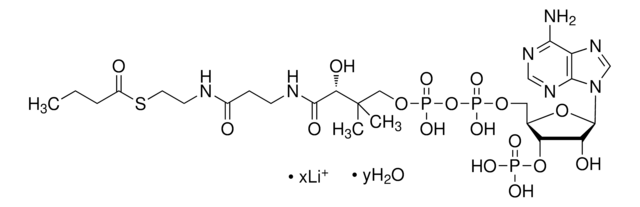
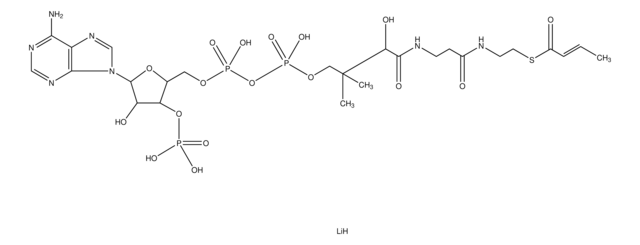
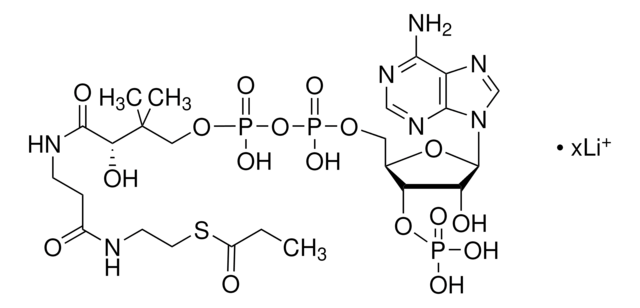
Myristoyl coenzyme A lithium salt
≥80.0% (HPLC), powder, protein myristoylation substrate
Synonym(s):
n-Tetradecanoyl Coenzyme A lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C35H62N7O17P3S · xLi+
CAS Number:
Molecular Weight:
977.89 (free acid basis)
UNSPSC Code:
12352202
NACRES:
NA.77
Recommended Products
product name
Myristoyl coenzyme A lithium salt, ≥80.0%
Assay
≥80.0%
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Myristoyl coenzyme A is a combination of coenzyme A and myristate. It serves as a substrate in protein myristoylation, catalyzed by the enzyme N-myristoyl transferase. Myristyolation involves the transfer of the myristoyl group to the glycine residue at the amino-terminal of the protein.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Substrates
Long chain fatty acid (C14) covalently linked to Coenzyme A. Substrate for de novo fatty acid synthesis. Fatty acylation has been shown to block G protein-associated calcium release by a direct allosteric modification of a component of the GTP-activated process.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Functional significance of myristoyl moiety in N-myristoyl proteins.
L J Knoll et al.
Methods in enzymology, 250, 405-435 (1995-01-01)
Signal Transduction - Single Cell Techniques, 327-327 (2008)
F Dittrich et al.
European journal of biochemistry, 252(3), 477-485 (1998-04-18)
Elongation of long-chain fatty acids was investigated in yeast mutants lacking endogenous de novo fatty acid synthesis. In this background, in vitro fatty acid elongation was dependent strictly on the substrates malonyl-CoA, NADPH and a medium-chain or long-chain acyl-CoA primer
K E Rys-Sikora et al.
The Journal of biological chemistry, 269(50), 31607-31613 (1994-12-16)
A sensitive and specific GTP-activated Ca2+ translocation process induces rapid Ca2+ movements within cells and appears to reflect G protein-induced membrane fusion or junctional communication between discrete subpopulations of Ca(2+)-pumping organelles (Ghosh, T. K., Mullaney, J. M., Tarazi, F. I.
C M D Swarbrick et al.
Acta crystallographica. Section D, Biological crystallography, 71(Pt 4), 986-995 (2015-04-08)
Acyl-CoA thioesterases catalyse the hydrolysis of the thioester bonds present within a wide range of acyl-CoA substrates, releasing free CoASH and the corresponding fatty-acyl conjugate. The TesB-type thioesterases are members of the TE4 thioesterase family, one of 25 thioesterase enzyme
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service