P8804
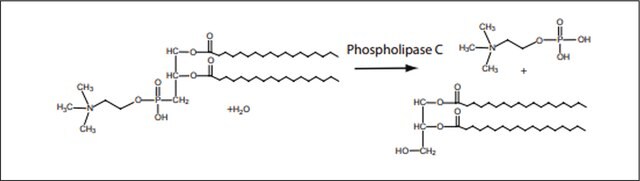
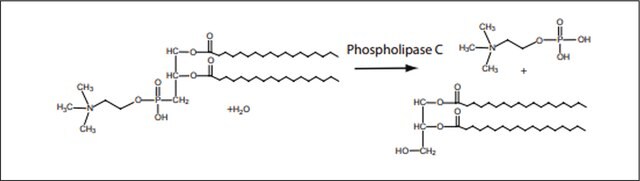
Phospholipase C, Phosphatidylinositol-specific from Bacillus cereus
buffered aqueous glycerol solution, ≥1,000 units/mg protein (Lowry)
Synonym(s):
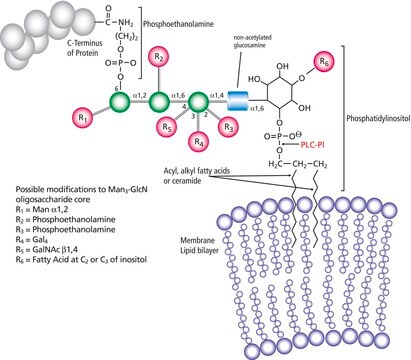
1-Phosphatidyl-D-myo-inositol inositol phosphohydrolase, cyclic-phosphate forming, PI-PLC
About This Item
Recommended Products
biological source
Bacillus sp. (Bacillus cereus)
Quality Level
form
buffered aqueous glycerol solution
specific activity
≥1,000 units/mg protein (Lowry)
mol wt
28 kDa
foreign activity
Phopholipase C (lecithinase) ≤1 units/mg protein
Sphingomyelinase ≤40 units/mg protein
storage temp.
2-8°C
Gene Information
Bacillus cereus E33L ... BCZK3513(3026815)
Looking for similar products? Visit Product Comparison Guide
General description
Application
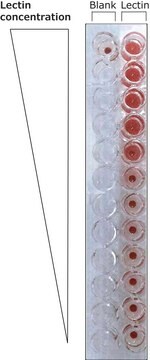
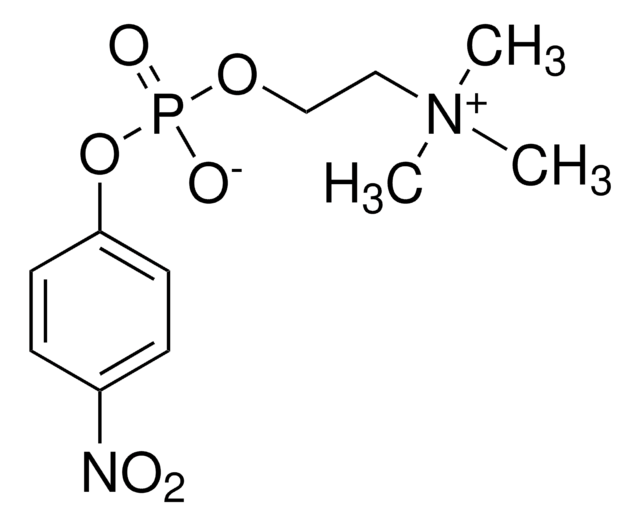
- in the hydrolysis of substrates p-nitrophenylphosphorylcholine (p-NPPC) and p-nitrophenylphosphorylphosphate (p-NPP)
- to cleave glycosylphosphatidylinositol (GPI) anchor of lynx1 protein and its detachment from plasma membrane
- to cleave immunolabeled HeLa cells
Biochem/physiol Actions
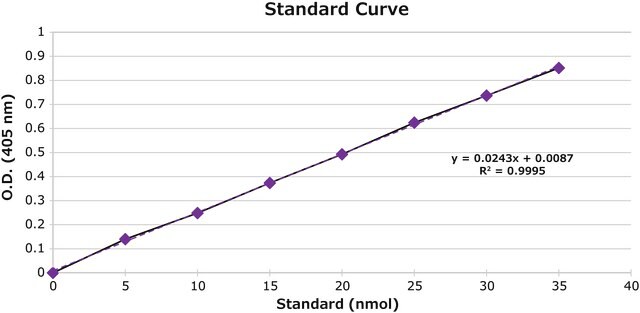
Unit Definition
Physical form
Analysis Note
inhibitor
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service