LC/MS (TOF) Analysis of Antiarrhythmic Drugs and Metabolites on Ascentis® Express HILIC
Materials
analytical column
standard
N-Desethylamiodarone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®(±)-Flecainide solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Lidocaine solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 μm particles (53939-U)
mobile phase
[A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile; (5:95, A:B, pH 7.0 with formic acid)
flow rate
0.4 mL/min
column temp.
35 °C
detector
ESI(+), full scan, m/z 200-800
injection
0.5 μL
sample
each compound, 300 ng/mL in 1% formic acid acetonitrile:water, 75:25
Description
Analysis Note
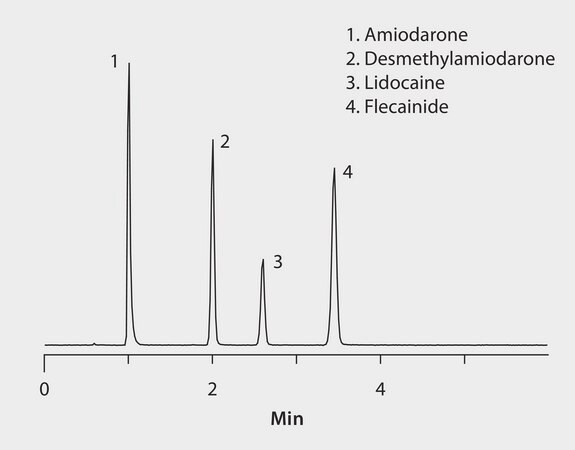
The basic nature of these compounds makes them targets for HILIC chromatographic separation. HILIC mobile phases consist of a high composition of acetonitrile, which facilitates the direct analysis of precipitated plasma samples without the need for additional sample solvent exchange. In most cases, the high organic mobile phase also facilitates increased analyte response with ESI(+) MS detection. Separation was performed on an Ascentis Express HILIC Fused-Core HPLC column. High purity solvents provided clean, robust operation. Cerilliant CRMs provided reliable quantification.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany