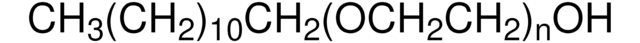235989
Brij® L4
average Mn ~362
Synonym(s):
Polyethylene glycol dodecyl ether, Polyoxyethylene (4) lauryl ether
About This Item
description
non-ionic
Quality Level
mol wt
average Mn ~362
refractive index
n20/D 1.451 (lit.)
density
0.95 g/mL at 25 °C (lit.)
HLB
9
SMILES string
CCCCCCCCCCCCOCCOCCOCCOCCO
InChI
1S/C20H42O5/c1-2-3-4-5-6-7-8-9-10-11-13-22-15-17-24-19-20-25-18-16-23-14-12-21/h21H,2-20H2,1H3
InChI key
WPMWEFXCIYCJSA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a surfactant for the synthesis of copper(I) oxide (Cu2O) spherical nanoparticles using a microemulsion technique
- as a non-ionic surfactant for preparing reverse micelles to synthesize Pt3Co alloy nanoparticles
- as a surfactant to enhance the efficiency of interfacial coupling of hydrophobic materials and to make hydrogel
Biochem/physiol Actions
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service