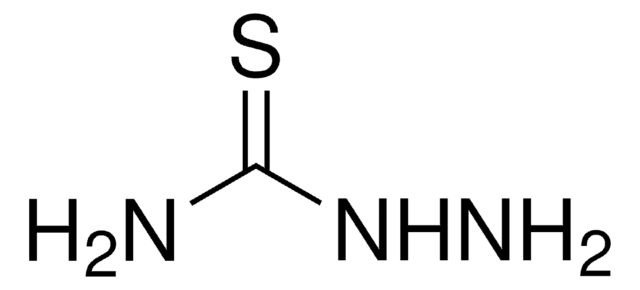
All Photos(2)
About This Item
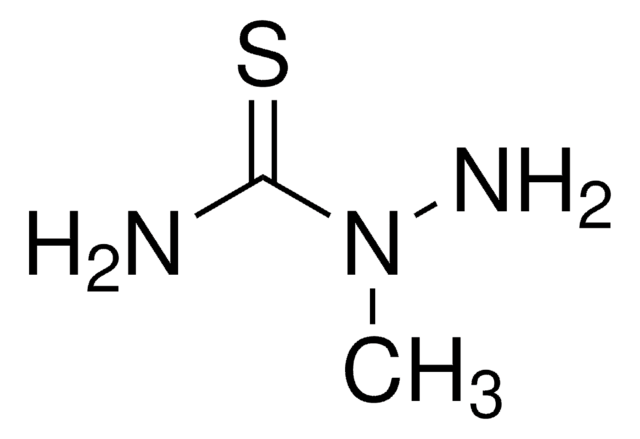
Linear Formula:
H2NNHCSN(CH3)2
CAS Number:
Molecular Weight:
119.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
153 °C (dec.) (lit.)
storage temp.
2-8°C
SMILES string
CN(C)C(=S)NN
InChI
1S/C3H9N3S/c1-6(2)3(7)5-4/h4H2,1-2H3,(H,5,7)
InChI key
FCPHVJQWZFNNKD-UHFFFAOYSA-N
General description
4,4-Dimethyl-3-thiosemicarbazide is alkyl derivative of thiosemicarbazide. Structure of 4,4-dimethyl-3-thiosemicarbazide in solution has been investigated by NMR and in solid state by IR and X-ray crystallographic methods.
Application
4,4-Dimethyl-3-thiosemicarbazide may be employed as chemical additive to investigate the corrosion inhibition of mild steel in acidic conditions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structures of anti, Z-4, 4-dimethyl-3-thiosemicarbazide, syn, E, Z-2, 4-dimethyl-3-thiosemicarbazide and syn, E-1-cyclopentano-3-thiosemicarbazone.
Valente EJ, et al.
Journal of Chemical Crystallography, 28(1), 27-33 (1998)
Molecular dynamics and quantum chemical calculation studies on 4, 4-dimethyl-3-thiosemicarbazide as corrosion inhibitor in 2.5 MH< sub> 2</sub> SO< sub> 4</sub>.
Musa AY, et al.
Materials Chemistry and Physics, 129(1), 660-665 (2011)
Fang Xie et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry (2018-07-15)
Copper 8-hydroxyquinoline-2-carboxaldehyde-thiosemicarbazide complex (CuHQTS) is a copper complex with strong anticancer activity against cisplatin-resistant neuroblastoma and prostate cancer cells in vitro by cell proliferation assay or fluorescent microscopic imaging. This study aimed to evaluate anti-prostate cancer activity of CuHQTS in
Liang Tian et al.
Biosensors & bioelectronics, 110, 110-117 (2018-04-01)
Enzyme mimics have been developed for bioassay of nucleic acids, with some of them involving complicated labeling. Herein, we report a label-free bioassay for ultrasensitive electronic determination of microRNA at an ultralow concentration based on target-triggered long-range self-assembly DNA-based hybridization
Franco Bisceglie et al.
Metallomics : integrated biometal science, 8(12), 1255-1265 (2016-11-15)
A comparative study between two bisthiosemicarbazones, 2,3-butanedione bis(4,4-dimethyl-3-thiosemicarbazone) and 2,3-butanedione bis(2-methyl-3-thiosemicarbazone), and their copper(ii) complexes is reported. The four compounds have been tested on a leukemia cell line U937 (p53-null) and on an adenocarcinoma cell line A549. The study includes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service