Nuclear Magnetic Resonance (NMR)
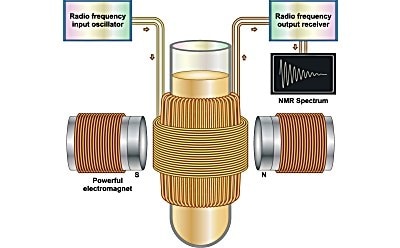
Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical technique used to determine the molecular structure and chemical composition of a sample. It works by analyzing the interaction of spinning nuclei in a strong magnetic field. In NMR spectroscopy, a stationary external magnetic field causes certain nuclei in a molecule to absorb selective radiofrequencies. The energy absorbed induces a transition in nuclear spins, which is observed on an NMR spectrum.
Featured Categories
Your Solvent Source: Find the right fit with Supelco®, SigmaAldrich®, & SAFC® brands, covering analytical, lab, & biopharmaceutical uses. Order online.
ISOTEC® Stable Isotopes are useful for tracer studies in proteomics and metabolomics, agents for MRI / MRS, and in a wide range of other biomedical applications.
Explore our selection of high-quality analytical reagents for wet chemistry, chromatography, spectroscopy, analytical, titration, water quality control, and electrochemistry.
Applications of NMR Spectroscopy
NMR spectroscopy is a non-destructive and non-invasive technique that is used to determine molecular structure and dynamics. The applications of NMR are diverse and include the following research areas and industries:
- In biology, NMR is applied to study macromolecules, such as proteins, lipids and nucleic acids. 13C, 1H, 15N, 31P, 23Na, and 19F are the most biologically relevant NMR-active nuclei, used to understand biochemical pathways involved in amino acid, lipid and carbohydrate metabolism.
- In chemistry, it’s widely used for both qualitative and quantitative analysis to monitor reactions, identify structures and assess purity.
- In polymer science, to analyze monomer ratio, molecular weight, tacticity, sequencing, chain length and branching, and to determine end groups.
- In the pharma industry, to determine the purity and quantity of active ingredients, excipients and impurities in pharmaceutical products
- In the petroleum industry, to assess hydrocarbons of raw petroleum and its products.
- In medicine, magnetic resonance imaging (MRI) is an application of NMR used for soft tissue analysis to identify the damaged or diseased tissues.
Principles of NMR Spectroscopy
Nuclear spin is related to the composition of an element’s nucleus. Nuclei containing an even number of both protons and neutrons have 0 nuclear spin and cannot undergo NMR (e.g., 4He,12C,16O). Nuclei with an odd number of protons and/or neutrons display nuclear spin and can experience NMR (e.g., 1H, 2H, 14N, 17O). These nuclei behave like tiny spinning magnets and can interact with an external magnetic field. Spinning nuclei also create their own magnetic field that can interact with other nuclei having spin.
A NMR instrument measures the interaction of nuclear spin states under the influence of a powerful magnetic field. The magnetic field causes nuclei to precess (rotate) like a spinning top. A precessing nucleus selectively absorbs energy from radiofrequency waves when the frequency of the precessing nuclei matches the low external frequency of radiofrequency waves interacting with it. When this absorption occurs, the precessing nucleus and radiofrequency waves are said to be in ‘resonance’, hence the term nuclear magnetic resonance. Resonance can be produced by tuning the frequency of the nuclei to the fixed frequency of radio waves, or by tuning the frequency of radio waves to that of the nuclei.
During NMR, an applied magnetic field excites nuclei having different magnetic moments across various energy levels. After absorbing a characteristic radiofrequency, the excited-state nuclei return to lower energy states by transferring energy to the surrounding environment. When energy is transferred to other atoms or the solvent, the relaxation process is called ‘spin-lattice relaxation’. If energy is transferred to neighboring nuclei at the same energy level, the process is termed ‘spin-spin relaxation’. These two relaxation processes are characterized by time constants: spin-lattice relaxation time (T1) and spin-spin relaxation time (T2), which are responsible for the resulting NMR spectrum.
Characteristics of a NMR Spectrum
A NMR spectrum is a plot of the applied radiofrequency against absorption. The position on the plot at which the nuclei absorbs is called the chemical shift. Chemical shift is affected by the electron density around the nucleus. If a nucleus is surrounded by a high electron density, the nucleus is shielded from the external magnetic field, which shifts the signals upfield on the NMR spectrum. If a nucleus is surrounded by an electronegative atom, it removes electron density around the nucleus and causes a ‘deshielding’ effect. This shifts the signal ‘downfield’ on an NMR spectrum. The spin of neighboring nuclei also affects the signals seen on an NMR spectrum and may cause splitting of the NMR signal, known as ‘spin-spin coupling’.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Reference or download our NMR shifts charts for the most common deuterated solvents. Proton NMR and carbon NMR tables aid chemists in separating signals of impurities that might originate from residual solvents or a reaction apparatus.
- NMR reference standards ensure proper instrument performance. Review key operational parameters for these standards such as pw90, sensitivity, resolution and line shape.
- Isotopic Labeling for NMR Spectroscopy of Biological Solids;
- Use this reference table to find the coupling values and chemical shifts of our NMR (deuterated) solvents. Melting and boiling points, molecular weight, density, and CAS number are also listed.
- The use of CRMs for the NMR quantification of phosphorylated organic compounds and metabolites offers several advantages as it is based on a signal comparison of the analyte with an internal or external reference standard.
- See All (15)
Related Protocols
- We presents an article regarding Polymer Analysis by NMR.
- Dr. Alan Robertson and Dr. Ben Davis at Vernalis, Ltd., UK, a provider of drug discovery and development services, have refined a novel protocol for the duallabelling of proteins using EnPresso® B Defined Nitrogen-free.
- See All (2)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?


