40302
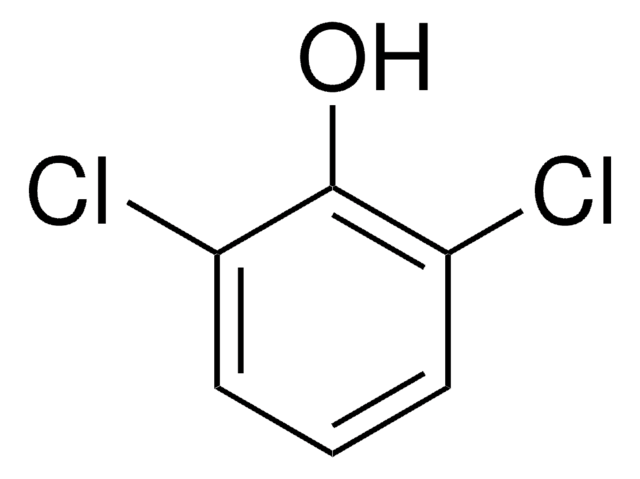
2,6-Dichlorophenol solution
certified reference material, 5000 μg/mL in methanol
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
product line
TraceCERT®
CofA
current certificate can be downloaded
packaging
ampule of 1 mL
concentration
5000 μg/mL in methanol
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
single component solution
storage temp.
2-8°C
InChI
1S/C6H4Cl2O/c7-4-2-1-3-5(8)6(4)9/h1-3,9H
InChI key
HOLHYSJJBXSLMV-UHFFFAOYSA-N
Application
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Separation of 2-Chlorophenol; 2,4-Dichlorophenol; 2,4,6-Tribromophenol; 2,4,6-Trichlorophenol; 2,4-Dinitrophenol; Pentafluorophenol; 2-Methylphenol, analytical standard; 2,3,4,6-Tetrachlorophenol; Pentachlorophenol; 4-Nitrophenol; 2-Bromophenol; 2,3,5,6-Tetrachlorophenol; 2,3,5-Trichlorophenol; 4-Chloro-3-methylphenol; 2,4,5-Trichlorophenol; 4-Methylphenol, analytical standard; 2,4-Dimethylphenol; 2-Nitrophenol; 3-Methylphenol, analytical standard; Phenol; 2-Methyl-4,6-dinitrophenol; 2,3,4-Trichlorophenol; 2,6-Dichlorophenol; 2,3,4,5-Tetrachlorophenol
Protocols
Chlorobenzilate; 4-Aminobiphenyl; 2-Fluorobiphenyl; N-Nitrosopyrrolidine; 1,2,4,5-Tetrachlorobenzene; 3-Methylcholanthrene; Phenacetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service