High-throughput Screening of Peptide Substrates for Tyrosine Kinases Using PEPscreen® Custom Peptide Library
Abstract
Protein Tyrosine Kinases (PTKs) play important roles in modulating a wide variety of cellular events, including differentiation, proliferation, metabolism and apoptosis. Those regulations are mediated by a series of signal cascades that involve phosphorylation of tyrosine residues in target proteins. Therefore, identification of the substrates for PTKs is very important for the studies in both basic research and drug development environment. Here, we report a high-throughput ELISA-based method to identify peptide substrates for class-specific and/or enzyme-specific PTKs that can be utilized for the detection of kinase activity both in vivo and in vitro. The amino acid sequences of peptide library containing 13-mer tyrosine peptides were generated by a predicting algorithm developed in-house for all potential protein substrates of PTKs identified from the public databases. Next, the peptides were synthesized using PEPscreen, a proprietary peptide synthesis platform, and then subjected to a screening of 39 PTKs using an ELISA-based method. The number of peptides selected for each PTK ranges from 2 to 71 with an average of 25 from a total of 376 peptides tested. The 173 sequences selected were classified into different groups with the reactivity to single, multiple, or all PTKs. The reactivity and specificity of the selected peptide substrates were further validated. The validation results indicated that this screening method has a very high sensitivity and reproducibility. Thus, the combination of our algorithm for selecting peptide sequences, the PEPscreen peptide synthesis platform, and ELISA-based assay method provide a successful high-throughput system for the screening of peptide substrates for many PTKs.
Materials and Methods
Unless otherwise indicated, all reagents and materials used in this work were obtained from Sigma-Aldrich (St. Louis, MO). The biotinylated peptides were synthesized on 96-well format (Sigma Genosys). The tyrosine kinases were obtained from SignalChem (Richmond, Canada), Upstate (Lake Placid, NY), and Invitrogen (Carlsbad, CA). Phosphorylated and unphosphorylated tyrosine peptides (Biotin-RRLIEDAEYAARG) were obtained from AnaSpec, Inc. (San Jose, CA). SigmaScreen™ Streptavidin-coated 384-well plate was used to anchor biotinylated peptides. Anti-phosphotyrosine monoclonal antibody, anti-mouse IgG alkaline phosphatase conjugate, and p-nitrophenyl phosphate liquid substrate system were used to detect phosphorylated tyrosine peptides. The optical density was obtained using SpectraMax Plus384 microplate spectrophotometer (Molecular Device, Sunnyvale, CA).
Selection of tyrosine peptides
The potential substrates of identified pharmaceutical important kinases were obtained by searching public databases (Phospho.ELM, and PhosphoSite). The FASTA sequence file including all the amino acid sequences of the substrates were then compiled and loaded onto KinasePhos database (no longer available) searching for the potential phosphorylation sites of each substrate. The output files were then modified by a computer program to identify the 13-mer peptides covering the potential phosphorylation sites. The commercially available peptide substrates for tyrosine kinases were used as the positive controls for screening.
Preparation of peptide library for screening
The peptides were reconstituted with 50% Acetonitrile at the final concentration of 5 mM. Four 96-well plates of peptides were reformatted into one 384-well plate. The 5 mM peptides were diluted to 0.5 mM with Tris-buffered saline (TBS) as working solutions for screening.
Kinase reaction
The kinase reaction buffer, the amounts of kinase and ATP for each reaction were used as indicated in the product information from the vendor. 2.5 μL of each 0.5 mM peptide was aliquoted into each well of a 384-well plate followed by adding 22.5 μL of kinase reaction mixture including kinase reaction buffer, ATP, and kinase. The plate was then incubated at 30 °C for 15 minutes with constant shaking of 120 rpm. 6 μL of 0.5 M EDTA was then added into each well to stop the kinase reaction.
ELISA assay
The kinase reaction solutions were transferred into a 384-well Streptavidin-coated plate followed by an incubation of one hour at room temperature with shaking. The solutions were then removed followed by three washes with 50 μL of Tris-buffered saline with TWEEN- 20 (TBST). 40 μL of mouse anti-phosphotyrosine antibody (1:2,000 dilution) was added into each well followed by an incubation of one hour at room temperature with shaking. The solutions were then removed followed by four washes with 50 μL of TBST. 40 μL of anti-mouse IgG conjugated alkaline phosphatase (1:30,000 dilution) was added into each well followed by an incubation of one hour at room temperature with shaking. The solutions were then removed followed by four washes with 50 μL of TBST. 40 μL of p-NPP was then added into each well followed by an incubation of 30 minutes at room temperature with shaking. 8 μL of 3 M sodium hydroxide was then added into each well to stop the enzymatic reaction. The optical absorptions at 405 nm were obtained by placing the plate into the plate reader.
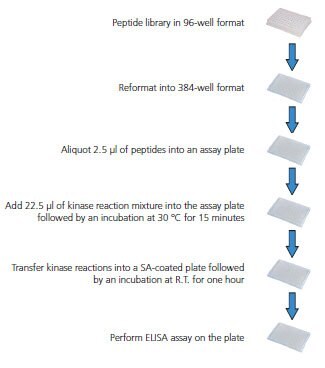
Figure 1.Overview of Screening Procedure
Results – Establishment and Optimization of Screening Platform
ELISA-based assay offers both sensitivity and selectivity for the screening of peptide substrates
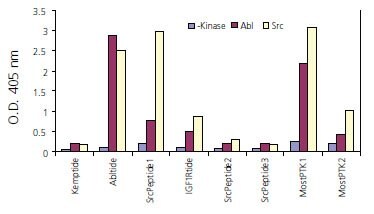
Figure 2.A total of nine biotinylated peptides were synthesized and the kinase assays were performed with Abl or Src kinase as described in Materials and Methods. The phosphorylated tyrosine residue was then detected by an ELISA-based assay using anti-phosphotyrosine antibidy. The results shown in the graph are the averages of two replicates. Kemptide: Biotin- LRRASLG-OH (a serine peptide as negative control); Abltide: Biotin-EAIYAAPFAKKK-OH; Src peptide1: Biotin-KVEKIGEGTYGVVYK-OH: IGF1Rtide: Biotin-KKKSPGEYVNIEFG-OH; Src peptide2: Biotin-IYGEF-OH: Src peptide3: Biotin-TSTEPQYQPGENL-OH: Most PTK1: Biotin-KKKGPWLEEEEEAYGWLDF-OH; Most PTK2: Biotin-RRLIEDAEYAARG-OH.
Optimization of ELISA-based assay platform

Figure 3.A A total of four biotinylated peptides were synthesized and then coated on the Streptavidin plate at 5 μg/mL, 1 μg/mL, 0.2 μg/mL, and 40 ng/mL. The ELISA-based assay was performed as described in Materials and Methods. Kemptide: Biotin-LRRASLG-OH; pS-Kemptide: Biotin-LRRApSLG-OH; Y-peptide: Biotin-RRLIEDAEYAARG-NH2; pY-peptide: Biotin-RRLIEDAEpYAARG-NH2. B 50 μL of pY-peptide (200 ng/mL) was used to coat Streptavidin plate. The phosphorylated tyrosine was detected by a series dilution of anti-phosphotyrosine antibody following the protocol described in Materials and Methods.
Results – Screening of Peptide Substrates for Thirty-nine Tyrosine Kinases
Primary Screening Results
Validation of Selected Peptides from Primary Screening
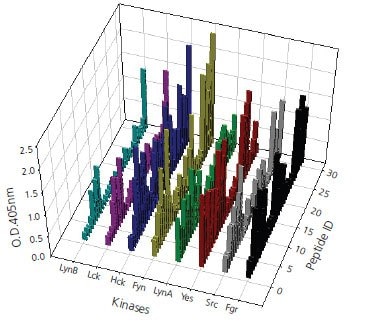
Figure 4.A total of 170 peptide substrates, divided in three groups based on different tyrosine protein kinases, have been selected after primary screening. Validation of peptide candidates was performed by ELISA assay with three repeats for each enzymatic reaction.This figure shows the validation results of activity assay for Tyrosine kinases of Src family against selected candidate peptides. The validation data are consistent and positively correlate more than 80% of results obtained in primary screening.
Conclusions
- The Algorithm developed in house for selecting peptide sequences works well as 50% of the peptides selected showed reactivity to single or multiple kinase.
- The synthesis of peptide library using PEPscreen method is very successful as only 1% of a total of 384 peptides failed.
- The ELISA-based assay offers both sensitivity and selectivity for the screening of peptide substrates for Tyrosine kinases.
To continue reading please sign in or create an account.
Don't Have An Account?