Organosilanols
Powerful Nucleophiles for Cross-Coupling
Over the past several years, the Pd-catalyzed cross-coupling of silicon compounds (Hiyama coupling) has rapidly gained acceptance as a suitable alternative to more commonly used methods such as Stille (Sn), Kumada (Mg), Suzuki (B), and Negishi cross-couplings (Zn).1 In particular, considerable progress has been made in the cross-coupling reactions of organosilanols and organosilanolates. Advantages of these organometallic nucleophiles include:
- Low toxicity
- Ease of preparation from inexpensive starting materials5
- Metal-halogen exchange or directed metallation
- Transition metal-catalyzed silylation of aryl halides
- Hydrosilylation
- Certain stoichiometry
- Robustness with respect to air and moisture stability
- Cross-coupling substrate scope
- Ability to cross-couple under fluoride or basic (via silanolate) activation
Alkenyl(dimethyl)silanol coupling: Preparation of diene and styrene derivatives
Using a fluoride activator, alkenyl(dimethyl)silanols readily couple with both aryl and alkenyl halides to give the coupled adducts in very good yield (Scheme 1).5 Alternatively, the Pd-catalyzed cross-coupling can be performed under basic activation using TMSOK for in situ generation of a nucleophilic silanolate.7-8 The utility of this performing the cross-coupling under basic activation, lies in the ability to couple in the presence of fluoride-sensitive silyl protecting groups as illustrated in Scheme 2.9 Alkynylsilanols have also been found to be active coupling partners under similar conditions.10


Both strategies, fluoride and base activation, were demonstrated in the total synthesis of the antifungal polyene macrolide RK-397.11 Specifically, the polyene segment of the natural product was prepared by sequential cross-coupling of a differentiable 1,4-bissilylbutadiene unit (Scheme 3). The dimethylsilanol moiety readily couples under basic activation (via a silanolate), while the other silyl substituent remains inert. Subsequent fluoride-promoted coupling of the benzyldimethylsilyl group provided the necessary tetraenoate linkage for completion of the target molecule.

Aryl(dimethyl)silanol coupling: Preparation of biaryls (and heterobiaryls)
Robust experimental protocols have been developed for biaryl coupling of aryl- and heteroaryl-(dimethyl)silanols with a variety of aryl iodides and bromides.13-14 Excellent yields were obtained in the coupling of (4-methoxyphenyl)dimethylsilanol with a diverse set of aryl halides using Cs2CO3 to generate the silanloate in situ (Scheme 4). Aryl bromides could be coupled using dppb as a ligand additive in toluene, while coupling of iodides was most effective using Ph3As in dioxane.

| Entry | R | X | Time (h) | Additive | Solvent | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 4-CO2Et | Br | 24 | dppb | toluene | 90 |
| 2 | 4-CH3 | Br | 18 | dppb | toluene | 90 |
| 3 | 4-H | Br | 12 | dppb | toluene | 85 |
| 4 | 4-OCH3 | Br | 18 | dppb | toluene | 92 |
| 6 | 2-CH3 | I | 24 | PhaAs | dioxane | 85 |
| 7 | 2-CF3 | I | 24 | PhaAs | dioxane | 82 |
| 8 | 2-NO2 | I | 24 | PhaAs | dioxane | 83 |
| 9 | 4-Ac | I | 3 | PhaAs | toluene | 91 |
Until recently, mild and general methods for cross-coupling of 2-heteroaryl organometallic nucleophiles were lacking. Boc-protected indoles are particularly challenging coupling substrates, owing to the decreased nucleophilicity at C-2. Typical procedures called for harsh reaction conditions (Stille coupling15) or failed to deliver the coupled product in acceptable yield (Suzuki coupling16-17). Denmark and co-workers have developed a set of general protocols for efficient coupling of these difficult substrates (Scheme 5). Both protocols call for generation of a sodium silanolate (basic activation). Silanolates generated in situ from NaOt-Bu undergo Pd-catalyzed cross-coupling with aryl iodides in the presence of CuI. Alternatively, silanolates generated in situ from NaH can be coupled without an additive.6 Finally, sodium dimethylsilanolates are also isolable and storable materials whose reactivity parallels that of in situ-formed silanolates.

| Entry | R | Base | Additive | Temp (o C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 4-NO2 | NaOt -Bu | Cul | rt | 6 | 84 |
| 2 | 4-CF3 | NaOt -Bu | Cul | rt | 22 | 82 |
| 3 | 2-OCH3 | NaOt-Bu | Cul | 50 | 24 | 75 |
| 4 | 4-OCH3 | NaOt-Bu | Cul | 50 | 24 | 72 |
| 5 | 4-CN | NaH | None | rt | 3 | 81 |
| 6 | 4-CO2Et | NaH | None | rt | 3 | 82 |
| 7 | 4-OCH3 | NaH | None | 80 | 3 | 68 |
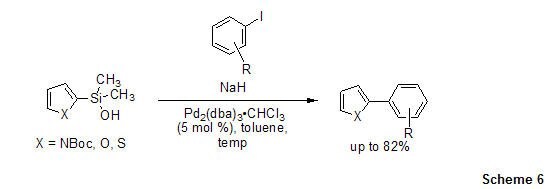
This methodology is applicable to cross-coupling of other heteroaryl(dimethyl)silanols with aryl iodides in the presence of Pd2(dba)3•CHCl3.6,19 Thiophene, furan, and pyrrole nucleophiles easily couple with both electron-rich and electron-deficient aryl iodides (Scheme 6).
Less expensive aryl bromides can be used in the reaction by changing to a highly-active Pd(I) catalyst developed by the Weissman and Moore groups (Scheme 7).19 The reactions times are generally shorter than with aryl iodides with no discernable decrease in yields. The Pd(I) catalyst displays very high activity that is comparable to or superior than many commonly used cross-coupling catalysts. It is readily prepared from (2-methylallyl)palladium(II) chloride dimer and P(t-Bu)3 in the presence of base.

To continue reading please sign in or create an account.
Don't Have An Account?