Hydrophilic Interaction Liquid Chromatography
The following pages contain an introduction to the HILIC separation technique and an overview of the strategies for developing robust methods for separation of polar and hydrophilic compounds.
What is HILIC?
Hydrophilic Interaction Liquid Chromatography
HILIC or Hydrophilic Interaction Liquid Chromatography is a high-performance liquid chromatographic (HPLC) technique for separation of polar and hydrophilic compounds. Originally the separation technique was called "Hydrophilic-Interaction Chromatography", and occasionally the expression "Aqueous Normal Phase" has also been used.
To put it simply: HILIC is a normal-phase type of separation but uses reversed-phase type eluents.
Thus, HILIC provides:
- A column with a hydrophilic stationary phase
- An eluent with water, buffer and a high concentration of water-miscible organic solvent.
A typical HILIC application uses acetonitrile at a concentration between 50-95% in an aqueous buffer such as ammonium formate, ammonium acetate or their acids, which have high solubility in organic solvents. HILIC can be used with many detection techniques, but when combined with electro spray ionisation mass spectrometry (ESI-MS) for example, HILIC will also enable higher sensitivities.
Reversed Elution Order Compared to RPLC
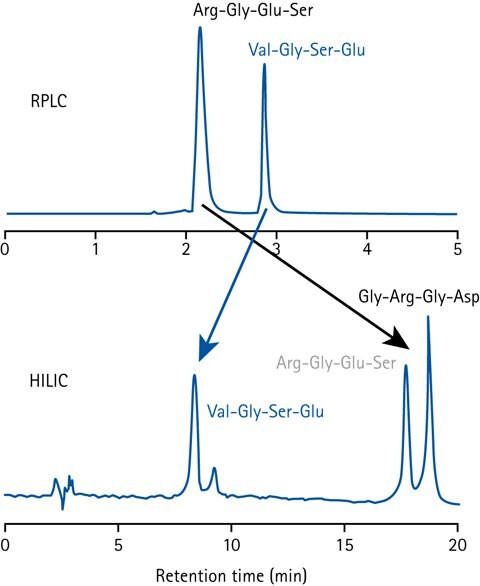
Figure 1.Comparison of peptide separation in HILIC and RPLC.
The elution order in HILIC is roughly the reverse of that in RPLC (Reversed-Phase Liquid Chromatography). A compound that elutes in the void volume on a RPLC column typically has high retention in HILIC, and vice versa.
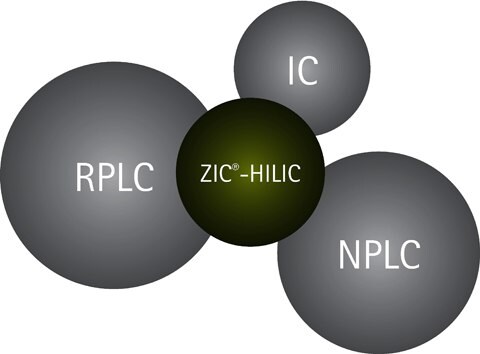
Figure 2.HILIC fills a gap in the chromatographic toolbox, but also partly overlaps with RPLC, NPLC, and IC separation techniques.
Compounds such as, acids, bases, ions, sugars, and other charged and neutral hydrophilic compounds that are troublesome to separate in RPLC, are much easier to separate in HILIC due to the different separation selectivity. Some compounds are possible to separate by more than one chromatographic technique, and HILIC does in that respect partly overlap both with RPLC, NPLC (Normal Phase Liquid Chromatography) and IC (Ion Chromatography)
Why Use HILIC?
Rapidly Increasing Interest for HILIC
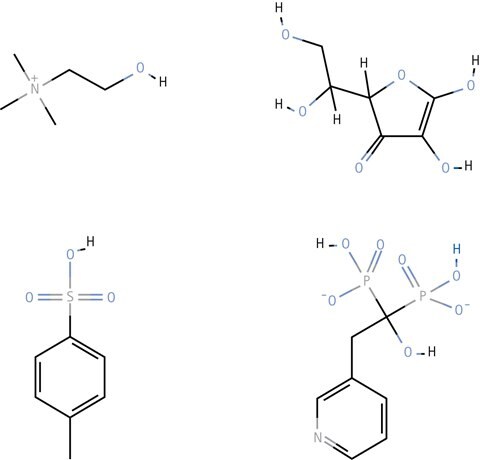
Figure 3.Example of compounds successfully separated using HILIC. Legend; Choline (top left), Ascorbic acid (top right), p-Toluenesulfonic acid (bottom left), and Risedronate (bottom right).
The HILIC separation technique is gaining much interest because it solves many of the previously difficult separation problems, including separation of small organic acids, basic drugs, cations, anions and many other neutral and charged substances.
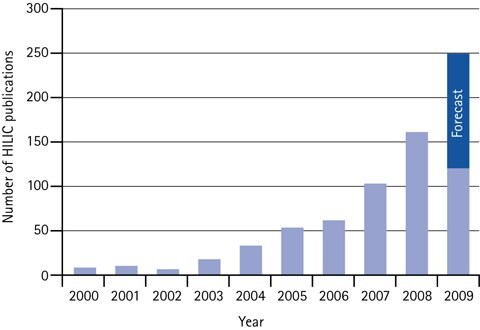
Figure 4.Annual number of scientific HILIC papers 2000 to mid of 2009, plus forecast for 2009.
The adoption of HILIC has resulted in an exponential increase in the number of citations within scientific papers.
Unattractive Alternatives
Due to the robustness and reproducibility of current bonded RPLC stationary phases, a common strategy has been to "tweak" the RPLC separation to encompass also polar and hydrophilic compounds. The techniques involved to accomplish this have been ion-pairing or micellar chromatography, polar embedded phases, and derivatization. These twists of RPLC have sometimes been able to get the job done, but they are also haunted by a number of limitations such as; low MS compatibility, low stationary phase stability and column bleeding, and time consuming with several interferences.
Robust Separation of Hydrophilic Compounds
HILIC is the most straightforward, versatile and robust separation technique for polar and hydrophilic compounds, compared to the above options. Dedicated, bonded stationary phases with high stability and reproducibility are since a few years available for HILIC separations, overcoming any previous limitations of HILIC relative to other separation techniques. In favour of HILIC is also the good solubility of polar and hydrophilic compounds in the water-containing HILIC eluents.
Sensitive Detection by LC-UV and LC/MS
HILIC separations are very easy to combine with several detection techniques, such as ultraviolet light absorbance (UV), fluorescence (FL), refractive index (RI), evaporative light scattering (ELSD), charged aerosol (CAD), and mass spectrometry (MS).
When HILIC is used with MS detection will the ESI sensitivity be much higher (10-100 times) compared to in RPLC. This is due to the high content of organic solvent in the mobile phase, which lowers surface tension, thereby simplifying the drop formation during the spray process. This significantly improves the formation of ions in the gas phase and thereby the sensitivity. Often, sample preparation can be simplified and made more efficient, which results in a further increase in sensitivity.
Orthogonality of HILIC
HILIC with RPLC is the Most Orthogonal Combination
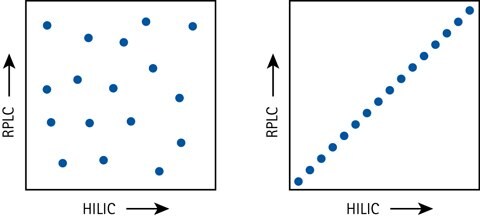
Figure 5.Separation orthogonality schematically expressed with HILIC and RPLC in a 2D separation space – excellent orthogonality (left) vs no orthogonality (right).
An attractive feature of HILIC is the 2D separation capabilities together with RPLC. HILIC-RPLC (or vice versa) is the most orthogonal combination of liquid separation techniques when orthogonality is defined as the coverage fraction of a 2D separation space. Other techniques such as IC and SEC (Size Exclusion Chromatography) results in more peak grouping or poorer resolution in comparison to HILIC.
More Information from the Same Sample
The benefits of the HILIC-RPLC orthogonality can also be utilized by running the same sample in both HILIC and RPLC mode and combining the data. This provides more information on the total sample, Figure 7, and can for example be used for enhanced protein identification via database searches or for approaches with sample fingerprint analysis.

Figure 6.More information from the same sample by combining data from both HILIC and RPLC, here exemplified with tryptic digest of BSA.
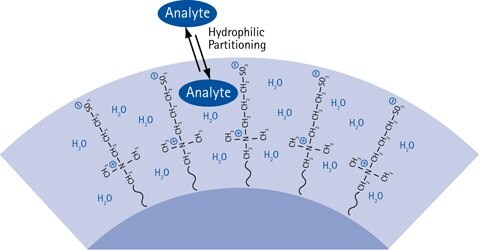
Figure 7.The HILIC partitioning process causing chromatographic retention.
Present HILIC theory dictates that HILIC retention is caused by a partitioning of the injected analyte solute molecules between the mobile phase eluent and a water-enriched layer in the hydrophilic HILIC stationary phase. The more hydrophilic the analyte is, the more is the partitioning equilibrium shifted towards the immobilized water layer in the stationary phase, resulting in more retention of the analyte.
Mechanism Still Debated
Although it is well established that a hydrophilic surface holds water when exposed to mixtures of organic solvent and water, the HILIC partitioning theory is only based on circumstantial evidence. There are studies pointing towards a more multimodal separation mechanism, involving hydrogen bonding as well as dipole-dipole interactions as important contributions. The selectivity differences observed for different HILIC stationary phases also indicate that more than one type of interaction is responsible for the chromatographic retention.
Fortunately, this lack of detailed mechanistic understanding does not limit the effectiveness of HILIC for separation of polar and hydrophilic compounds.
Adjusting the Retention
The retention and selectivity in HILIC is affected by adjusting the eluent by varying the fraction (and type) of organic solvent, the concentration (and type) of buffer, and the pH. Retention increases with increasing fraction of organic solvent, and this is the most effective way of tuning retention.

Figure 8.Relative approximate importance of different eluent factors for adjusting retention in HILIC.
The eluent buffer and pH affects retention of ionized and ionisable molecules more than neutrals, because an ionized molecule is more hydrophilic, and thus is retained more strongly in HILIC. The effect of column temperature in HILIC separations is often rather small (typically less than in RPLC), but ultimately this depends on the nature of the retained molecule.
More information on the aspects of the HILIC mechanism and the effect of different solvents and buffer types on the separation can be found in the booklet "A Practical Guide to HILIC".
HILIC Stationary Phases
There is More to HILIC than HILIC
Since HILIC only requires a hydrophilic surface to adsorb and immobilise a water layer for the partitioning process, it can be tempting to assume that all hydrophilic stationary phase surfaces are suitable for HILIC and also have the same performance. This is certainly not the case! The overall charge, the pH-dependence, the extent of secondary interactions and the phase stability can differ a lot between different types of HILIC stationary phases. These characteristics will affect the separation selectivity and also the chance of succeeding with the development of a robust HILIC method.
Classification of Stationary Phases for HILIC
In principle HILIC stationary phases can be divided into three different groups – neutral, charged and zwitterionic:
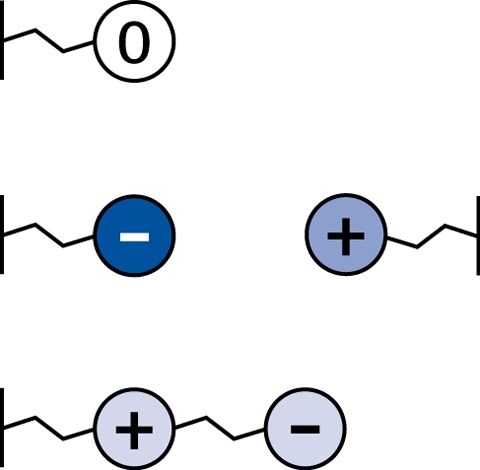
Figure 9.Different classes of stationary phases for HILIC. Neutral (top), charged (middle), and zwitterionic (bottom).
Neutral
- no electrostatic interactions
- Diol phases, amide phases
Charged
- strong electrostatic interactions
- Plain silica phases, aminopropyl phases
Zwitterionic
- weak electrostatic interactions
- SeQuant® ZIC®-HILIC ZIC®-HILIC and ZIC®-pHILIC phases
Good and Bad Secondary Interactions
With stationary phases that change their properties as a function of pH (such as plain silica), there is a complex relationship between the retention and the pH, making it harder to predict and optimize the separation.
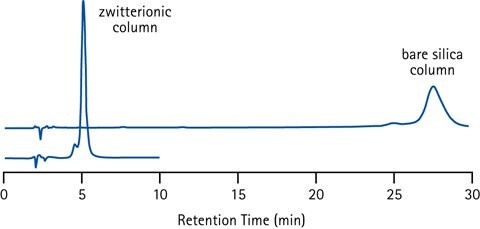
Figure 10.Retention of the basic and strongly positively charged peptide Bradykinin (pI=12) on different HILIC stationary phases (plain silica and zwitterionic) illustrating the effect of too strong ionic interactions. Conditions; 40% acetonitrile in ammonium acetate buffer (50 mM, pH 6.5).
Separation selectivity between different analytes can be favoured by the electrostatic (ionic) interactions that are contributing to the retention with charged HILIC stationary phases. With too strong electrostatic interactions high concentrations of salt ions in the eluent is required to disrupt these interactions for efficient elution. This is disadvantageous, since high salt or buffer concentrations reduce the sensitivity in several detection techniques - including MS. Balancing the stationary phase charge by using a bonded zwitterion is the design strategy employed in the Merck's SeQuant® ZIC®-HILIC columns to get the best of both worlds.
Solvents for HILIC Eluents
Acetonitrile and Other Polar Organic Solvents
Acetonitrile is typically the first choice as the organic solvent component for HILIC due to the good miscibility with water, the good HILIC retention, and the low viscosity. However, many other polar organic solvents such as methanol, ethanol, propanol, isopropanol, dioxane, acetone and DMF can also be used as the non-eluting solvent in HILIC eluents. Relative solvent strengths in HILIC is approximately:
acetone < iso-propanol ~ propanol < acetonitrile < ethanol < dioxane < DMF ~ methanol < water
Controlling Retention with Solvent
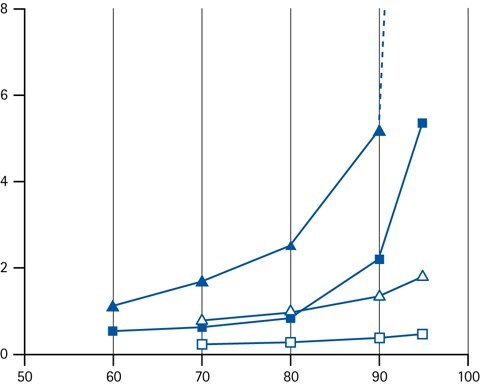
Figure 11.Retention factor k as a function of the concentration of acetonitrile (closed symbols) or methanol (open symbols) as organic modifier in the eluent. Legend; (triangles) cytosine (squares) adenine.
The most effective way of changing retention if HILIC is by adjusting the concentration of the organic solvent component. In HILIC, the retention increase with increasing fraction of organic solvent.
It has been recognized though, that the relationship between the retention factor (k) and the fraction of organic solvent in the mobile phase do not follow a logarithmic relationship (which typically is the case in RPLC). Instead it is normally a log-log relationship that is observed. The practical consequence of this is that HILIC gradients should be run shallower, because the retention changes more with a small adjustment of organic solvent concentration.
To continue reading please sign in or create an account.
Don't Have An Account?