Cultrex® 3-D Culture Matrix™ Reduced Growth Factor Basement Membrane Extract, PathClear® Protocol
Product No. 3445-010-01
I. Product Description
3D Culture is a cell culture method that provides cells with the necessary structure and signaling cues to direct reconstruction of the tissue architecture, and these methods provide physiologically predictive in vitro models for evaluating development and disease. Normal cells assemble into organoids that structurally resemble their emanating tissues, exhibit a polarized morphology, undergo cell cycle regulation, and produce tissue specific proteins.1-6 While cancer cells assemble into tumor-like structures, lacking an organized architecture or cell cycle regulation and exhibiting tumor-specific markers depending on the extend of malignancy.7-9
To aid in the advancement of this technology, we present Cultrex® 3-D Culture Matrix™ RGF BME which is the first Basement Membrane Extract produced and qualified specifically for use in 3D culture studies. The 3-D Culture Matrix™ RGF BME provides the foundation for cells to grow in three dimensions allowing for the formation of structures in vitro. To provide the most standardized basement membrane extract for use in 3D cultures, a special process is employed to reduce growth factors and manufacture matrix at a standard concentration of approximately 15 mg/mL. The efficacy of this product is then evaluated in a 3D culture for validation.
II. Specifications
- Concentration: 14 - 16 mg/mL.
- Source: Murine Engelbreth-Holm-Swarm (EHS) tumor
- Storage buffer: Dulbecco’s Modified Eagle’s medium without phenol red (D5030), with 10 μg/mL gentamicin sulfate (G1264)
III. Reagents And Equipment Required But Not Provided
- Equipment
- Laminar flow hood
- 37 °C CO2 incubator
- Low speed swinging bucket 4 °C centrifuge and tubes for cell harvesting
- Hemocytometer or other means to count cells
- –20 °C storage
- Ice bucket
- Pipettes and pipette aid
- Bright Field Microscope with 4X objective and digital camera
- Reagents
- Disposables
IV. Precautions and Limitations
- For Research Use Only. Not for use in diagnostic procedures.
- The physical, chemical, and toxicological properties of these products may not yet have been fully investigated; therefore, we recommend the use of gloves, lab coats, and eye protection while using these chemical reagents.
V. Material Qualification
- Functional Assays
- Tube formation assay – BME promotes formations of capillary-like structures by human (HBMVEC, HUVEC) or mouse (SVEC4-10) endothelial cells.
- 3D culture – Basement Membrane Extract promotes differentiation of a human epithelial cell line derived from mammary gland (MCF-10A) or human prostate (PC-3) into acinar structures.
- Sterility Testing
- PathClear® – Negative by PCR test for mycoplasma; 17 bacterial and virus strains typically included in mouse antibody production (MAP) testing, plus 13 additional murine infectious agents including LDEV, for a total of 31 organisms and viruses.
- No bacterial or fungal growth detected after incubation at 37 ⁰C for 14 days following USP sterility testing guidelines.
- Endotoxin concentration ≤8 EU/mL by LAL assay
- Gelling Assay
- BME gels in less than 30 minutes at 37 ⁰C, and maintains the gelled form in culture medium for a minimum of 14 days at 37 ⁰C.
VI. Storage and Stability
Product is stable for a minimum of 3 months from date of shipment when stored at –20 ⁰C in a manual defrost freezer. For optimal stability, store at –80 ⁰C. Avoid freeze-thaw cycles.
VII. 3D Culture Protocol
This procedure must be conducted in an aseptic environment, such as a laminar flow hood or clean room, using aseptic technique to prevent contamination. This protocol is based on Debnath et al.1 It is provided as a well characterized example; different cell lines may require different cell culture conditions and incubation periods.
- Culture cells as recommended by cell supplier to establish a stable population at 37 ⁰C in a CO2 incubator; growth media, growth factors, serum requirements, and incubation period may vary by cell type: e.g. MCF-10A (DMEM, 5% Horse Serum (HS), 20 ng/mL hEGF, 500 ng/mL Hydrocortisone, 100 ng/mL Cholera Toxin, 10 μg/mL Insulin, 1X Pen/Strep) and PC-3 (RPMI-1640, 10% HS, 5% Fetal Bovine Serum (FBS).
- Thaw 3-D Culture Matrix™ RGF BME at 2-8 ⁰C overnight.
- Working on ice, add 250 μL of 3-D Culture Matrix™ RGF BME per well of a sterile 48 well plate, incubate the plate at 37 ⁰C for 30 minutes to promote gelling of matrix.
- Working on ice, add 98 mL of growth media (as recommended by cell supplier and 2 mL of 3-D Culture Matrix™ RGF BME (final concentration of 2%) to a sterile container, and label this container “Assay Media,” and swirl to mix. Any unused 3-D Culture Matrix™ RGF BME can be stored at 4 ⁰C up to one week or stored in working aliquots at –20 ⁰C in a manual defrost freezer.
- Incubate Assay Media at 37 ⁰C for 30 minutes in preparation for cell dilution.
- Harvest cells from culture, and dilute cells to 1 x 104 cells/mL in 24 mL of Assay Medium.
- Add 500 μL of cell suspension to each well of the 48 well plate containing 3-D Culture Matrix™ RGF BME.
- Incubate plate at 37 ⁰C in a CO2 incubator overnight.
- Each day, observe cell growth and structure formation via inverted microscope, and replace 48 well in a CO2 incubator overnight at 37 ⁰C.
- On day 4, carefully pipet off old media using a sterile serological pipette, and replace with new Assay Media. Repeat on day 8 and day 12.
- When structures have grown to desired size, prepare cells for analysis (as recommended by manufacturer), and analyze structures. This point is dependent on cell line and growth conditions. In our qualification, MCF-10A cells are analyzed at 16 days, and PC-3 cells are analyzed at 10 to 12 days.
Recommendations for Analysis
- To fix cells, incubate for 20 minutes in 2% formalin, 1X PBS at room temperature.
- Cells may be analyzed in the plate on BME; they may be transferred to a microscope slide (very carefully); or they may be embedded in paraffin and sectioned.
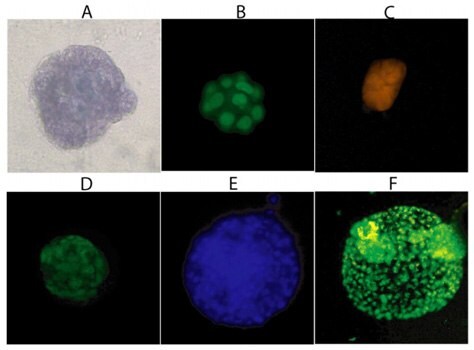
Figure 1.Three-Dimensional Cellular Structures. Staining of MCF-10A cells after sixteen days is 3-D Culture Matrix™ RGF BME with: A) Cell Staining Kit (structural), B) SYBR® Green (nuclear), and C) MitoShift™ (mitochondrial potential); and staining of PC-3 cells after twelve days in3-D Culture Matrix™ RGF BME with: D) Calcein AM (cell viability), E) CPA dye 1 (nuclear) and F) Depsipher™ (mitochondrial potential)
Legal Information
3-D Culture Matrix is a trademark of Trevigen, Inc.
Cultrex and PathClear are registered trademarks of Trevigen, Inc.
SYBR is a registered trademark of Molecular Probes, Eugene OR
Depsipher and MitoShift are trademarks of Trevigen, Inc.
References
To continue reading please sign in or create an account.
Don't Have An Account?