All Photos(1)
About This Item
Empirical Formula (Hill Notation):
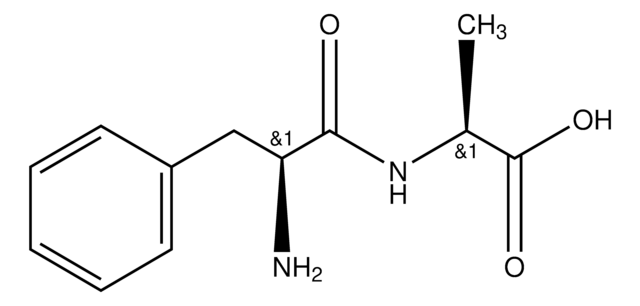
C8H16N2O3
CAS Number:
Molecular Weight:
188.22
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
Leu-Gly,
Assay
≥98% (TLC)
form
powder
color
white
storage temp.
−20°C
SMILES string
CC(C)C[C@H](N)C(=O)NCC(O)=O
InChI
1S/C8H16N2O3/c1-5(2)3-6(9)8(13)10-4-7(11)12/h5-6H,3-4,9H2,1-2H3,(H,10,13)(H,11,12)/t6-/m0/s1
InChI key
LESXFEZIFXFIQR-LURJTMIESA-N
Biochem/physiol Actions
Leucylglycine (Leu-Gly) is used to study the kinetics and speciation of copper(II)- cis, cis-1,3,5-triaminocyclohexane complex-promoted hydrolysis of dipeptides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cleavage of L-leucine-containing dipeptides by Clostridium butyricum.
Khelifa N, Brik M, Tessedre AC, et al.
Bioorganic & Medicinal Chemistry, 9, 109-112 (1999)
Irene L Gutiérrez et al.
Molecular neurobiology, 55(10), 7872-7885 (2018-02-27)
The decline in brain noradrenaline levels is associated with the progression of certain neurodegenerative diseases. This seems to be due, at least in part, to the ability of noradrenaline to limit glial activation and to reduce the damage associated with
Yuki Fujii et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 7(7-8), 843-851 (2002-08-31)
The hydrolysis of glycylglycine (GylGly), glycyl-L-leucine (GlyLeu), L-leucylglycine (LeuGly) and glycyl-DL-serine (GlySer) promoted by a copper(II)- cis, cis-1,3,5-triaminocyclohexane complex [Cu(II)TACH] was investigated at 70 degrees C and pH 7-10, using HPLC. The observed pseudo-first-order rate constants (k(obs)) and rate enhancing
Yu-Hsin Lin et al.
Nutrients, 10(10) (2018-10-03)
Hot water was used to obtain Chlorella sorokiniana hot water extract (HWE). Subsequently, this byproduct was freeze-dried, hydrolysed at 50 °C using Protease N to obtain C. sorokiniana protein hydrolysates (PN-1), and then digested with a gastrointestinal enzyme (PN-1G). The
Yu-Hsin Lin et al.
Nutrients, 11(6) (2019-06-19)
This research focuses on cobia skin hydrolysates and their antihypertensive effects via the inhibitory activities of angiotensin I-converting enzyme (ACE). Marine fish Cobia (Rachycentron canadum) skin was hydrolysed for 5 h using Protamex and Protease N to obtain the cobia
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service