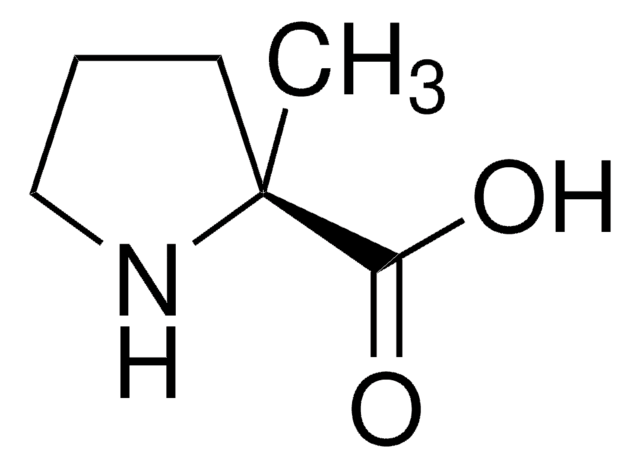
391204
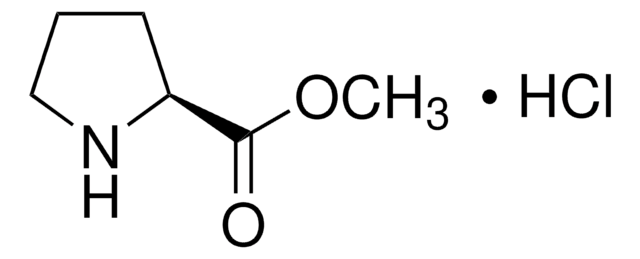
Methyl pipecolinate hydrochloride
97%
Synonym(s):
Methyl 2-piperidinecarboxylate hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H13NO2 · HCl
CAS Number:
Molecular Weight:
179.64
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
205 °C (dec.) (lit.)
SMILES string
Cl.COC(=O)C1CCCCN1
InChI
1S/C7H13NO2.ClH/c1-10-7(9)6-4-2-3-5-8-6;/h6,8H,2-5H2,1H3;1H
InChI key
APCHKWZTSCBBJX-UHFFFAOYSA-N
General description
Methyl pipecolinate hydrochloride is a hydrochloride salt of methyl piperidine-2-carboxylate (methyl pipecolinate). The kinetics of the enzymatic separation of enantiomeric forms of methyl pipecolinate using Candida antarctica Lipase A (CAL-A) has been reported. Its role as catalyst for the standard Diels-Alder reaction has been examined.
Application
Reactant for synthesis of:
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Advances in the kinetic and dynamic kinetic resolution of piperazine-2-carboxylic acid derivatives with Candida antarctica lipase A; structural requirements for enantioselective N-acylation.
Hietanen A, et al.
ARKIVOC (Gainesville, FL, United States), 5, 60-74 (2012)
Aldehyde-based racemization in the dynamic kinetic resolution of N-heterocyclic a-amino esters using Candida Antarctica lipase A.
Liljeblad A, et al.
Tetrahedron, 60(3), 671-677 (2004)
The α-effect in cyclic secondary amines: new scaffolds for iminium ion accelerated transformations.
Brazier JB, et al.
Tetrahedron, 65(48), 9961-9966 (2009)
Enantioselective lipase-catalyzed reactions of methyl pipecolinate: transesterification and N-acylation.
Liljeblad, A, et al.
Tetrahedron Letters, 43(13), 2471-2474 (2002)
Alkoxycarbonylpiperidines as N-nucleophiles in the palladium-catalyzed aminocarbonylation.
Takacs A, et al.
Monatshefte fur Chemie / Chemical Monthly, 145(9), 1473-1478 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service