推荐产品
等級
pharmaceutical primary standard
API 家族
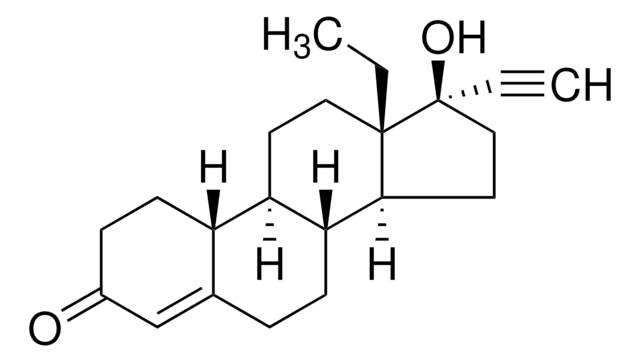
levonorgestrel
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
CC[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1CC[C@@]2(O)C#C
InChI
1S/C21H28O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,13,16-19,23H,3,5-12H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
InChI 密鑰
WWYNJERNGUHSAO-XUDSTZEESA-N
基因資訊
human ... PGR(5241)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Levonorgestrel USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Carc. 2 - Lact. - Repr. 1A
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
The efficacy of levonorgestrel intrauterine systems for endometrial protection: a systematic review.
Y-L Wan et al.
Climacteric : the journal of the International Menopause Society, 14(6), 622-632 (2011-10-25)
Oral progestogens are commonly used for endometrial protection in women at higher risk of developing endometrial abnormality. Long-term intrauterine progestogens may offer an attractive alternative to oral therapy. To review the evidence regarding the efficacy of intrauterine levonorgestrel-releasing systems (LNG-IUS)
Richard F Lowe et al.
Contraception, 87(4), 486-496 (2012-11-06)
The use of intrauterine devices as a contraceptive method has been steadily growing in developing countries. Anemia in reproductive-age women is a growing concern in those settings. A systematic review of studies with measured hemoglobin and serum ferritin at baseline
Paul D Blumenthal et al.
International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 112(3), 171-178 (2011-01-29)
To review the literature for economic and health-related quality of life outcomes data associated with the use of the levonorgestrel-releasing intrauterine system (LNG-IUS) in the management of heavy menstrual bleeding. We searched the MEDLINE and EMBASE databases simultaneously using the
Ayman A A Ewies et al.
Obstetrical & gynecological survey, 67(11), 726-733 (2012-11-16)
Endometrial hyperplasia is a commonly seen gynecological condition that affects women of all age groups. Whereas hysterectomy is the most preferred treatment option for complex endometrial hyperplasia with atypia, there is no consensus regarding the first-line management of women with
Lisa L Bayer et al.
The Journal of adolescent health : official publication of the Society for Adolescent Medicine, 52(4 Suppl), S54-S58 (2013-04-03)
The levonorgestrel intrauterine system (LNG-IUS) is an underused contraceptive method in adolescent populations. In addition to being a highly effective, reversible, long-acting contraception, the LNG-IUS has many noncontraceptive health benefits including reduced menstrual bleeding, decreased dysmenorrhea and pelvic pain related
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门