推荐产品
化驗
≥90% (LC/MS-ELSD)
形狀
solid
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
−20°C
SMILES 字串
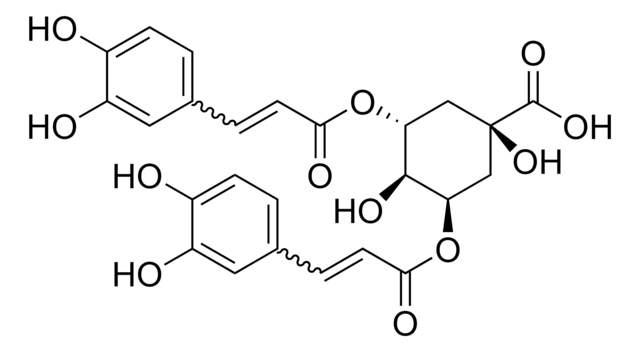
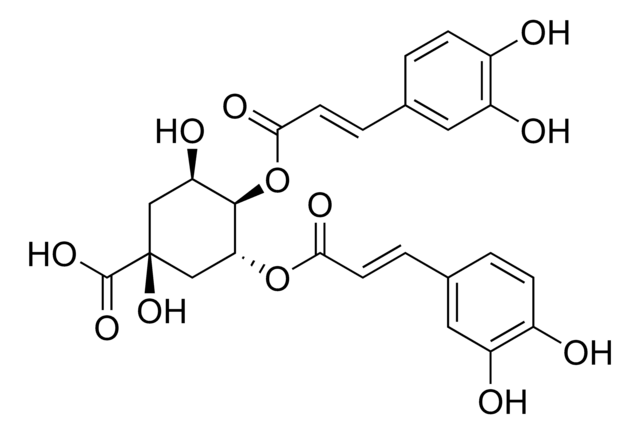
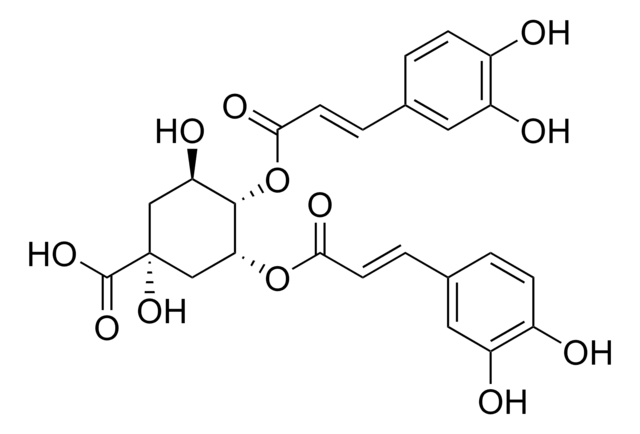
O[C@@H]1C[C@](O)(C[C@@H](OC(=O)\C=C\c2ccc(O)c(O)c2)[C@@H]1OC(=O)\C=C\c3ccc(O)c(O)c3)C(O)=O
InChI
1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(31)36-20-12-25(35,24(33)34)11-19(30)23(20)37-22(32)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-30,35H,11-12H2,(H,33,34)/b7-3+,8-4+/t19-,20-,23-,25+/m1/s1
InChI 密鑰
UFCLZKMFXSILNL-PSEXTPKNSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
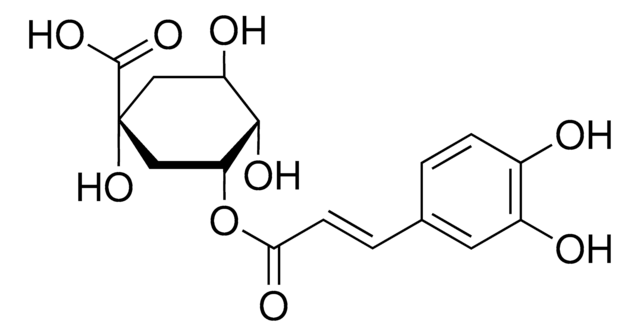
3,4-Di-O-caffeoylquinic acid (3,4-COQ) is a component of the medicinal herb Youngia japonica.It has a caffeoyl moiety attached with a hexacyclic quinic acid in its structure.
應用
3,4-Di-O-caffeoylquinic acid has been used as a reference standard:
- to quantify the caffeoylquinic acids of Artemisia frigida Willd. (Fringed sagewort) using high-performance liquid chromatography with diode array detection and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-DAD-ESI-QQQ-MS)
- to quantify the phenolic compounds of Artemisia species using high-performance liquid chromatography with diode array detection (HPLC-DAD) technique
- for metabolic profiling of methanolic leaf extract of A. nallamalayana and to identify the phenolic acids using High-Performance Liquid Chromatography-Ultraviolet (HPLC-UV) analysis
生化/生理作用
3,4-Di-O-caffeoylquinic acid, along with 3,5-dicaffeoylquinic acid and luteolin-7-O-glucoside, is a component of the medicinal herb Youngia japonica. It has known antiviral activity and along with 3,5,-dicaffeoylquinic acid, shows activity against respiratory syncyntial virus (RSV). 3,4-COQ elicits antioxidant effects which mediate its neuroprotective effects against in vitro retinal damage. It also exerts cytoprotective effects against oxidative damage in bone marrow-derived mesenchymal stem cells (bmMSCs).
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Esterification of the Free Carboxylic Group in 3,4-di-O-caffeoylquinic Acid Enhances the Inhibition Activity against Respiratory Syncytial Virus (RSV)
Wu D, et al.
Medicinal Chemistry (2015)
Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin.
Li Y, et al.
Antiviral Research, 68(1), 1-9 (2005)
Miki Hiemori-Kondo et al.
Bioscience, biotechnology, and biochemistry, 84(3), 621-632 (2019-11-26)
The antioxidant activity of Petasites japonicus flower buds cultivated in Tokushima, Japan, was examined in vitro and in vivo. The flower bud extracts were assayed using either oxygen radical absorbance capacity or 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. Antioxidants in the
A comparative assessment of in vitro cytotoxic activity and phytochemical profiling of Andrographis nallamalayana JL Ellis and Andrographis paniculata (Burm. f.) Nees using UPLC-QTOF-MS/MS approach
Goel N, et al.,
Royal Society of Chemistry Advances, 11(57), 35918-35936 (2021)
Yoshimi Nakajima et al.
Life sciences, 80(4), 370-377 (2006-10-19)
We investigated whether water extract of Brazilian green propolis (WEP) and its main constituents [caffeoylquinic acid derivatives (3,4-di-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, chlorogenic acid) and cinnamic acid derivatives (p-coumaric acid, artepillin C, drupanin, baccharin)] exert neuroprotective effects against the retinal damage
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门