About This Item
推荐产品
生物源
Fusarium sp. (Fusarium moniliforme)
品質等級
化驗
≥98% (HPLC)
形狀
powder
溶解度
methanol: 9.80-10.20 mg/mL, clear, colorless to light yellow
儲存溫度
2-8°C
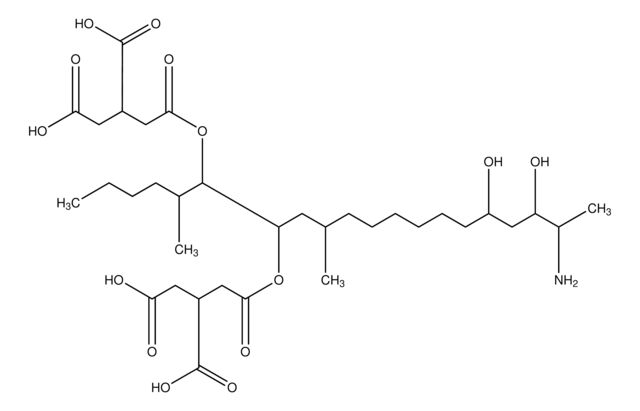
SMILES 字串
CCCC[C@@H](C)[C@@H](OC(=O)C[C@@H](CC(O)=O)C(O)=O)[C@H](C[C@@H](C)C[C@H](O)CCCC[C@@H](O)C[C@H](O)[C@H](C)N)OC(=O)C[C@@H](CC(O)=O)C(O)=O
InChI
1S/C34H59NO15/c1-5-6-9-20(3)32(50-31(44)17-23(34(47)48)15-29(41)42)27(49-30(43)16-22(33(45)46)14-28(39)40)13-19(2)12-24(36)10-7-8-11-25(37)18-26(38)21(4)35/h19-27,32,36-38H,5-18,35H2,1-4H3,(H,39,40)(H,41,42)(H,45,46)(H,47,48)/t19-,20+,21-,22+,23+,24+,25+,26-,27-,32+/m0/s1
InChI 密鑰
UVBUBMSSQKOIBE-DSLOAKGESA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
伏马菌素B1(FB1)与鞘氨醇结构相似。 因此,伏马菌素B1可以抑制神经酰胺合成酶(鞘氨醇N-乙酰基转移酶),其可导致神经酰胺生物合成和复杂鞘脂生物合成的中断。
應用
- 对从成年猪盲肠内容物中的真菌毒素进行分析的标准品
- 研究伏马菌素B1对朊病毒蛋白(PrP)异构体的代谢和羊瘙痒病朊病毒蛋白PrPSc合成的影响,以及
- 研究伏马毒素B1对猪空肠外植体的毒性作用。
生化/生理作用
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Carc. 2 - Repr. 2 - STOT RE 2
標靶器官
Kidney,Liver
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门