推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. T1410000
traceable to USP 1655000
API 家族
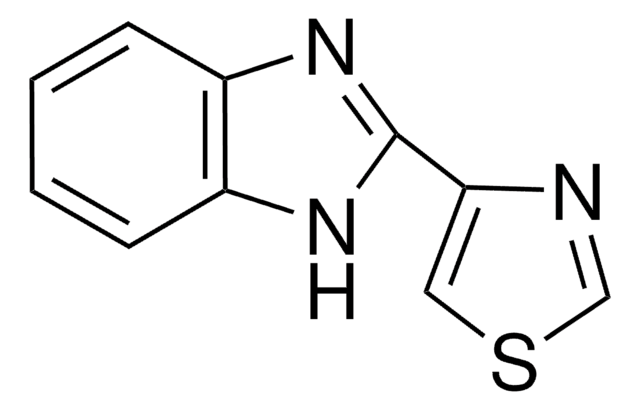
thiabendazole
CofA
current certificate can be downloaded
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-30°C
SMILES 字串
c1ccc2[nH]c(nc2c1)-c3cscn3
InChI
1S/C10H7N3S/c1-2-4-8-7(3-1)12-10(13-8)9-5-14-6-11-9/h1-6H,(H,12,13)
InChI 密鑰
WJCNZQLZVWNLKY-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Thiabendazole is an orally effective broad spectrum anthelmintic and antimycotic drug with limited side-effects.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Thiabendazole may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations and preparations by spectrophotometric, spectrofluorimetric and chromatography techniques.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA9062 in the slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
訊號詞
Warning
危險聲明
防範說明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole--linear ion trap in different types of water in Serbia
Petrovic M, et al.
The Science of the Total Environment, 468(2), 415-428 (2014)
Studies on the toxicologic and pharmacologic properties of thiabendazole
Robinson HJ, et al.
Toxicology and Applied Pharmacology, 7(1), 53-63 (1965)
Development of a spectrofluorimetric method for determination of thiabendazole in tablets
Kucukkolbasi S and Kilic E
Journal of Applied Pharmaceutical Science, 3(2), 19-19 (2013)
Mechanism of action of the fungicide thiabendazole, 2-(4?-thiazolyl) benzimidazole
Allen PM and Gottlieb D
Applied and Environmental Microbiology, 20(6), 919-926 (1970)
Suely R C Andrade et al.
Journal of pharmaceutical and biomedical analysis, 33(4), 655-665 (2003-11-19)
Three multivariate calibration methods, Principal Component Regression (PCR), the K-matrix method and Q-mode factor analysis followed by varimax and Imbrie's oblique rotations were applied to the simultaneous spectrophotometric determinations of mebendazole (MBZ)-cambendazole (CBZ) and thiabendazole (TBZ)-mebendazole in commercial samples of
实验方案
GC Analysis of Agricultural Pesticides (Standard) on SLB®-5ms
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门