推荐产品
品質等級
產品線
Novabiochem®
化驗
≥98.0% (HPLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
官能基
Fmoc
儲存溫度
2-8°C
InChI
1S/C31H25NO5/c33-29(21-8-2-1-3-9-21)22-16-14-20(15-17-22)18-28(30(34)35)32-31(36)37-19-27-25-12-6-4-10-23(25)24-11-5-7-13-26(24)27/h1-17,27-28H,18-19H2,(H,32,36)(H,34,35)/t28-/m0/s1
InChI 密鑰
SYOBJKCXNRQOGA-NDEPHWFRSA-N
一般說明
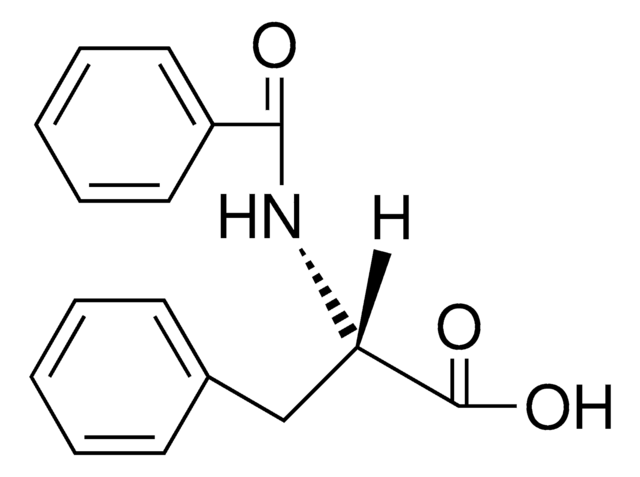
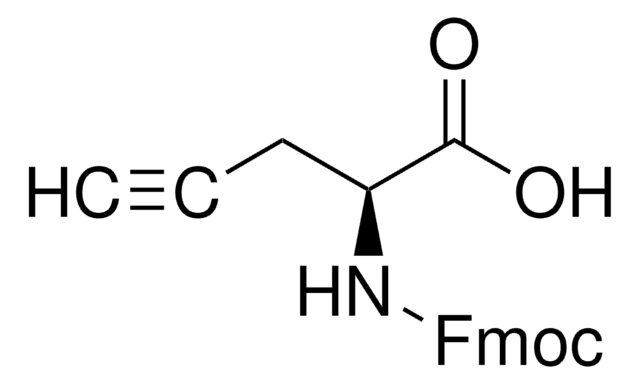
Fmoc-p-Bz-Phe-OH-OH is a useful tool for preparing photoactivatable peptide-based affinity probes [1]. On photolysis at 366 nm, Benzoylphenylalanine (Bpa) generates a biradical that has a preference for insertion into C-H bonds, particularly those of Leu, Val and Met side chains. The derivative can be introduced using standard coupling method such as PyBOP and is stable to conditions used in peptide chain extension. For the cleavage of Bpa containing peptide from the resin, the use of thiols and silanes should be avoided as dithioketal formation and reduction, respectively, have been observed.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] K. T. O′Neil, et al. (1989) J. Biol. Chem., 264.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] K. T. O′Neil, et al. (1989) J. Biol. Chem., 264.
分析報告
Colour (visual): white to slight yellow to beige
Appearance of substance (visual): powder
Identity (IR): passes test
Optical rotation α 25/D (c=1 in DMF): -31.0 - -27.0 °
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Appearance of substance (visual): powder
Identity (IR): passes test
Optical rotation α 25/D (c=1 in DMF): -31.0 - -27.0 °
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
法律資訊
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门