推荐产品
化驗
98%
形狀
liquid
折射率
n20/D 1.571 (lit.)
bp
196 °C (lit.)
mp
−57 °C (lit.)
密度
0.989 g/mL at 25 °C (lit.)
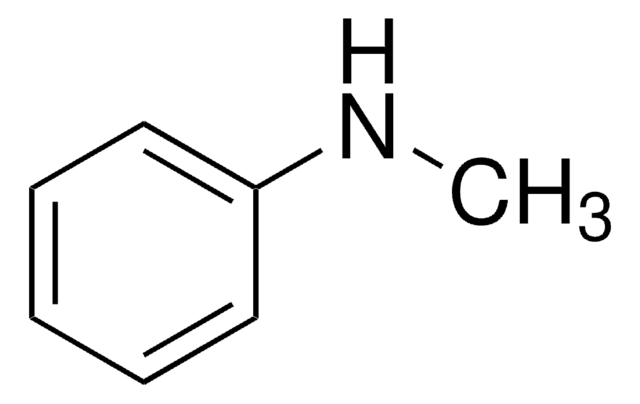
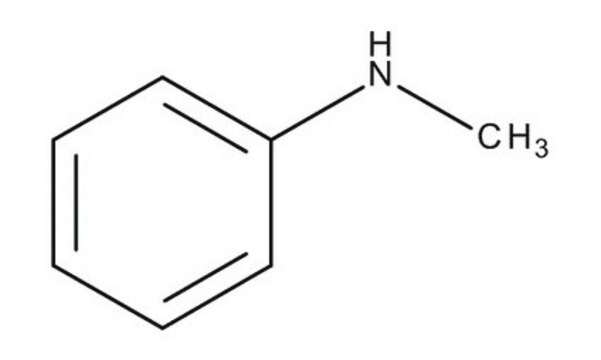
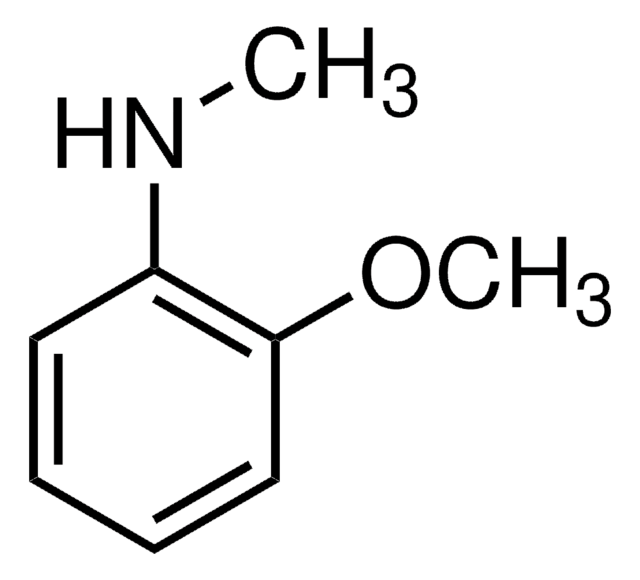
SMILES 字串
CNc1ccccc1
InChI
1S/C7H9N/c1-8-7-5-3-2-4-6-7/h2-6,8H,1H3
InChI 密鑰
AFBPFSWMIHJQDM-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N-甲基苯胺是用于以下方面的通用试剂:
- 针对铑催化不对称硼氢化制备亚磷酰胺配体。
- 作为C3选择性吲哚甲酰化的甲酰化试剂。
- 合成了一种基于红质的荧光探针,HKGreen-3,用于检测过亚硝酸根。
- 作为合成AKT(蛋白激酶B)抑制剂-IV的关键起始物料。
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - STOT RE 2 Oral
標靶器官
Liver,spleen,Bone marrow
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
185.0 °F - closed cup
閃點(°C)
85 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Efficient amide-directed catalytic asymmetric hydroboration.
Smith S M, et al.
Journal of the American Chemical Society, 130(12), 3734-3735 (2008)
Synthesis and biological evaluation of analogues of AKT (protein kinase B) inhibitor-IV.
Sun Q, et al.
Journal of Medicinal Chemistry, 54(5), 1126-1139 (2011)
HKGreen-3: a rhodol-based fluorescent probe for peroxynitrite.
Peng T and Yang D
Organic Letters, 12(21), 4932-4935 (2010)
V Arjunan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 72(2), 436-444 (2008-12-17)
The Fourier transform infrared (FTIR) and FT-Raman spectra of 2-chloro-4-methylaniline and 2-chloro-6-methylaniline have been measured in the range 4000-400 and 4000-100cm(-1), respectively. Utilising the observed FTIR and FT-Raman data, a complete vibrational assignment and analysis of the fundamental modes of
Dongmei Li et al.
Dalton transactions (Cambridge, England : 2003), (2)(2), 291-297 (2008-12-18)
The mechanism of N-dealkylation of N-cyclopropyl-N-methylaniline () catalyzed by cytochrome P450 (P450) was investigated using density functional theory. This reaction involves two steps. The first one is a Calpha-H hydroxylation on the N-substituent to form a carbinolaniline complex, and the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门