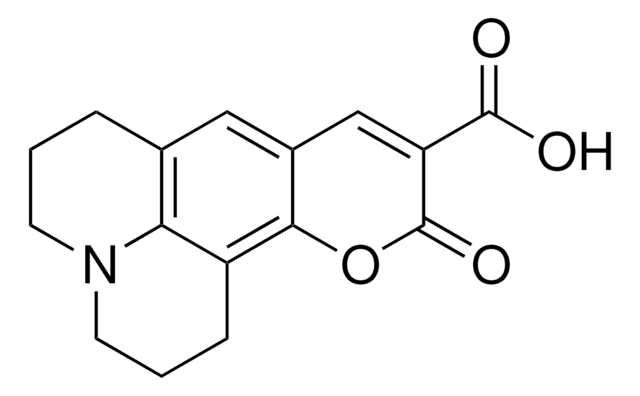
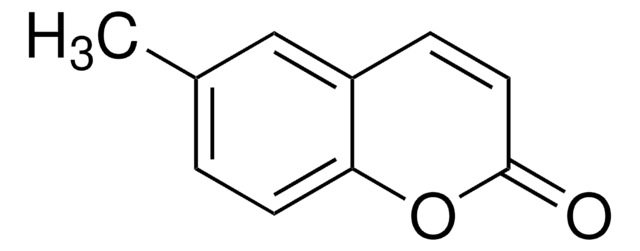
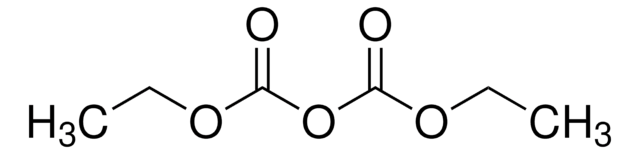
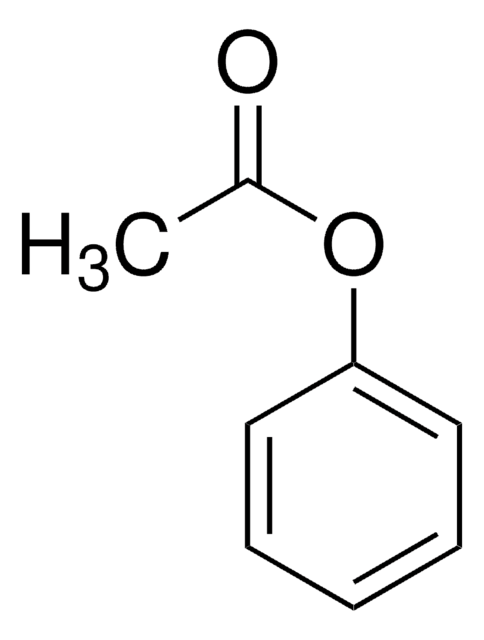
推荐产品
化驗
99%
形狀
liquid
折射率
n20/D 1.556 (lit.)
bp
272 °C (lit.)
mp
24-25 °C (lit.)
密度
1.169 g/mL at 25 °C (lit.)
SMILES 字串
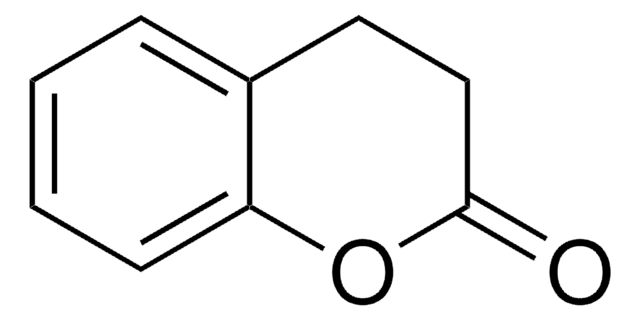
O=C1CCc2ccccc2O1
InChI
1S/C9H8O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-4H,5-6H2
InChI 密鑰
VMUXSMXIQBNMGZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Skin Sens. 1
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
>212.0 °F - closed cup
閃點(°C)
> 100 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Katrin Häser et al.
Journal of agricultural and food chemistry, 54(17), 6236-6240 (2006-08-17)
Natural dihydrocoumarin, which is of great interest in the flavor industry, was biotechnologically produced from pure coumarin or tonka bean meal with Pseudomonas orientalis, Bacillus cereus, and various Saccharomyces cerevisiae strains. Coumarin was shown to be converted to melilotic acid
Tomohiro Doura et al.
Chemical communications (Cambridge, England), 48(10), 1565-1567 (2011-05-18)
The first dual activatable hypochlorite ((-)OCl)-sensing probe was developed, based on a new proof-of-concept design involving signal-activatable (1)H chemical probes using the triple-resonance NMR technique. The probe enabled fluorescence-(1)H MR dual turn-on detection of (-)OCl in solution and in crude
Sharona Shachan-Tov et al.
Free radical biology & medicine, 49(10), 1516-1521 (2010-08-31)
Afri et al. reported in this journal (Free Radic. Biol. Med.32:605-618; 2002) that a direct relationship exists between the depth of alkanoylcoumarins 1 within the liposomal lipid bilayer and the rate at which they undergo superoxide-mediated saponification. These results were
The flavoring agent dihydrocoumarin reverses epigenetic silencing and inhibits sirtuin deacetylases.
Andrew J Olaharski et al.
PLoS genetics, 1(6), e77-e77 (2005-12-20)
Sirtuins are a family of phylogenetically conserved nicotinamide adenine dinucleotide-dependent deacetylases that have a firmly established role in aging. Using a simple Saccharomyces cerevisiae yeast heterochromatic derepression assay, we tested a number of environmental chemicals to address the possibility that
Jakub Modranka et al.
Bioorganic & medicinal chemistry, 20(16), 5017-5026 (2012-07-14)
A series of new 3-methylidenechroman-2-ones bearing various aromatic moieties and various substituents at position 4 were synthesized in a three step reaction sequence. Friedel-Crafts alkylation of phenols or naphthols using ethyl 3-methoxy-2-diethoxyphosphorylacrylate in the presence of trifluoromethanesulphonic acid gave 3-diethoxyphosphorylchromen-2-ones.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门