所有图片(1)
About This Item
经验公式(希尔记法):
C5H2Cl3N
CAS号:
分子量:
182.44
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
75-77 °C (dec.) (lit.)
SMILES 字串
Clc1cncc(Cl)c1Cl
InChI
1S/C5H2Cl3N/c6-3-1-9-2-4(7)5(3)8/h1-2H
InChI 密鑰
KKWRVUBDCJQHBZ-UHFFFAOYSA-N
一般說明
3,4,5-Trichloropyridine is a pyridine derivative. It undergoes oxidation with trifluoroacetic anhydride (TFAA) in the presence of hydrogen peroxide urea complex.
應用
3,4,5-Trichloropyridine may be employed as a secondary standard in quantitative NMR (qNMR) studies.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
A practical, efficient, and rapid method for the oxidation of electron deficient pyridines using trifluoroacetic anhydride and hydrogen peroxide-urea complex.
Caron S, et al.
Tetrahedron Letters, 41(14), 2299-2302 (2000)
Torgny Rundlöf et al.
Journal of pharmaceutical and biomedical analysis, 52(5), 645-651 (2010-03-09)
In quantitative NMR (qNMR) selection of an appropriate internal standard proves to be crucial. In this study, 25 candidate compounds considered to be potent internal standards were investigated with respect to the ability of providing unique signal chemical shifts, purity
Torgny Rundlöf et al.
Journal of pharmaceutical and biomedical analysis, 93, 111-117 (2013-11-12)
Standards are required in quantitative NMR (qNMR) to obtain accurate and precise results. In this study acetanilide was established and used as a primary standard. Six other chemicals were selected as secondary standards: 3,4,5-trichloropyridine, dimethylterephthalate, maleic acid, 3-sulfolene, 1,4-bis(trimethylsilyl)benzene, and
Shi Shen et al.
Journal of pharmaceutical and biomedical analysis, 114, 190-199 (2015-06-13)
This study investigated the accuracy of the quantitative NMR method for purity determination of ACE inhibitors reference standards and the discovery of two pairs of new diastereoisomers. Six types of ACE inhibitors, imidapril hydrochloride, benazepril hydrochloride, lisinopril, enalapril maleate, quinapril
Monika Johansson et al.
Journal of pharmaceutical and biomedical analysis, 100, 215-229 (2014-08-30)
An effective screening procedure to identify and quantify active pharmaceutical substances in suspected illegal medicinal products is described. The analytical platform, consisting of accurate mass determination with liquid chromatography time-of-flight mass spectrometry (LC-QTOF-MS) in combination with nuclear magnetic resonance (NMR)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门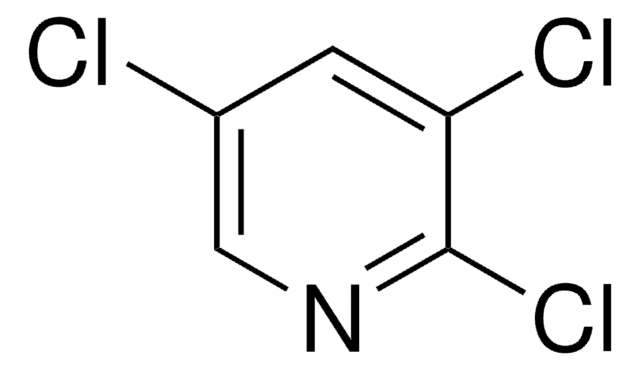


![4,4'-diamino[1,1'-biphenyl]-2,2'-disulfonic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/132/504/f9fd296f-c246-427d-9118-23f7b80e2be1/640/f9fd296f-c246-427d-9118-23f7b80e2be1.png)