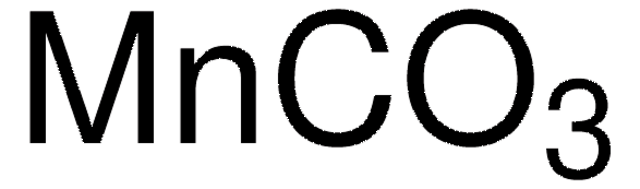推荐产品
化驗
≥99.9% trace metals basis
形狀
powder
雜質
≤1,000.0 ppm Trace Metal Analysis
mp
>200 °C (lit.)
溶解度
dilute aqueous acid: slightly soluble(lit.)
密度
3.12 g/mL at 25 °C (lit.)
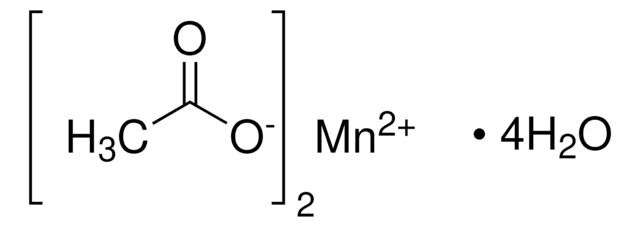
SMILES 字串
[Mn++].[O-]C([O-])=O
InChI
1S/CH2O3.Mn/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI 密鑰
XMWCXZJXESXBBY-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- A primary electrode material in asymmetric supercapacitors for improving the charge storage capacity and overall performance of the supercapacitors.
- A precursor for synthesizing manganese oxide, which is used as a component in various electrochemical applications, including batteries and supercapacitors.
- A precursor material in the synthesis of oxygen vacancy-rich nitrogen-doped manganese carbonate (MnCO2@N) microspheres. These microspheres are then employed as cathode materials in aqueous zinc-ion batteries (ZIBs) to enhance their electrochemical performance.
- A potential electrocatalyst for the oxygen evolution reaction (OER) in water splitting applications.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
商品
Recently, layer-by-layer (LbL) assembly has emerged as a versatile, gentle and, simple method for immobilization of functional molecules in an easily controllable thin film morphology.1,2 In this short review, we introduce recent advances in functional systems fabricated by using the mild, yet adaptable LbL technique.
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门