所有图片(2)
About This Item
线性分子式:
BrCH2CH2CHBrCOCl
CAS号:
分子量:
264.34
Beilstein:
4955401
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
等級
technical
化驗
~90% (GC)
折射率
n20/D 1.535
密度
2.00 g/mL at 20 °C
儲存溫度
2-8°C
SMILES 字串
ClC(=O)C(Br)CCBr
InChI
1S/C4H5Br2ClO/c5-2-1-3(6)4(7)8/h3H,1-2H2
InChI 密鑰
WYZLYWUZERABRL-UHFFFAOYSA-N
應用
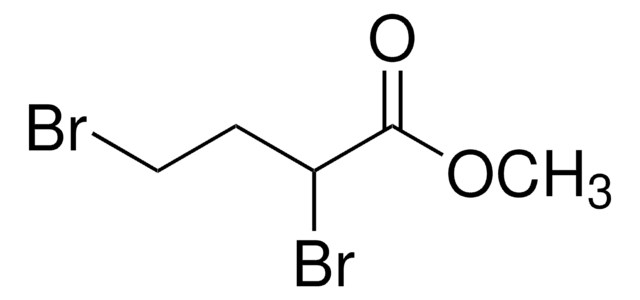
2,4-Dibromobutyryl chloride was used in the preparation of:
- methyl 2,4-dibromobutanoate
- series of 3-(3, 5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones, anti-inflammatory/analgesic agents
- α-bromolactam
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Preparation of enantiopure 2-acylazetidines and their reactions with chloroformates.
Ma S, et al.
Tetrahedron Letters, 48(2), 269-271 (2007)
Bhooma Raghavan et al.
The Journal of organic chemistry, 71(5), 2151-2154 (2006-02-25)
A concise, stereoselective synthesis of alpha-substituted gamma-lactams is reported. gamma-Lactam scaffolds 2 and 3, possessing an Evans' chiral auxiliary and two types of N substituents, were successfully alkylated with different electrophiles to give alpha-substituted gamma-lactams with reasonable diastereoselectivities. The use
H Ikuta et al.
Journal of medicinal chemistry, 30(11), 1995-1998 (1987-11-01)
A series of 3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones was synthesized and evaluated as candidate antiinflammatory/analgesic agents as well as dual inhibitors of prostaglandin and leukotriene synthesis. Some compounds that showed dual inhibitory activity were found to possess equipotent antiinflammatory activities to indomethacin, with reduced
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门