推荐产品
品質等級
化驗
99%
形狀
solid
mp
108-110 °C (lit.)
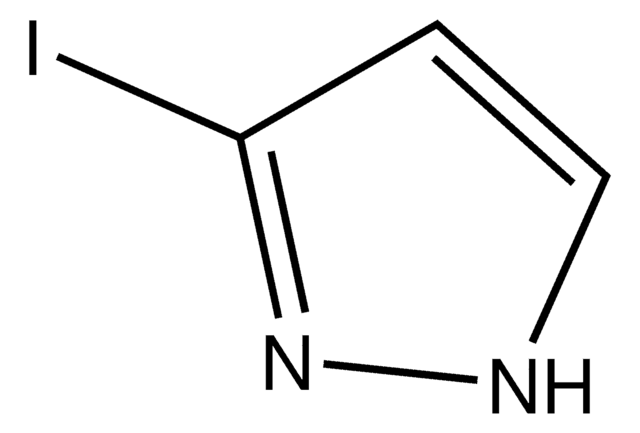
SMILES 字串
Ic1cn[nH]c1
InChI
1S/C3H3IN2/c4-3-1-5-6-2-3/h1-2H,(H,5,6)
InChI 密鑰
LLNQWPTUJJYTTE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
用于铟介导的杂二芳烃合成。
應用
4-Iodopyrazole was used in an indium-mediated synthesis of heterobiaryls.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Metin Zora et al.
The Journal of organic chemistry, 76(16), 6726-6742 (2011-07-12)
Electrophilic cyclizations of α,β-alkynic hydrazones by molecular iodine were investigated for the synthesis of 4-iodopyrazoles. α,β-Alkynic hydrazones were readily prepared by the reactions of hydrazines with propargyl aldehydes and ketones. When treated with molecular iodine in the presence of sodium
A Kojo et al.
Biochemical pharmacology, 42(9), 1751-1759 (1991-10-09)
Pyrazole and several of its derivatives increase the hepatic microsomal coumarin 7-hydroxylase to a variable extent. The strongest inducers are pyrazole itself and those derivatives which have a hydroxy group or a halogen at the 4-position of the molecule. The
Green iodination of pyrazoles with iodine/hydrogen peroxide in water.
Kim MM, et al.
Tetrahedron Letters, 49(25), 4026-4028 (2008)
Enrique Font-Sanchis et al.
The Journal of organic chemistry, 72(9), 3589-3591 (2007-04-05)
The palladium-mediated coupling reaction between triorganoindium reagents and organic electrophiles is extended to the synthesis of heteroaromatic compounds. Both electron-rich and electron-poor heterocycles can act as the organic electrophile or as the organoindium derivative.
Some iodinated pyrazole derivatives.
D Giles et al.
Journal of the Chemical Society. Perkin transactions 1, 13, 1179-1184 (1966-01-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门