All Photos(1)
About This Item
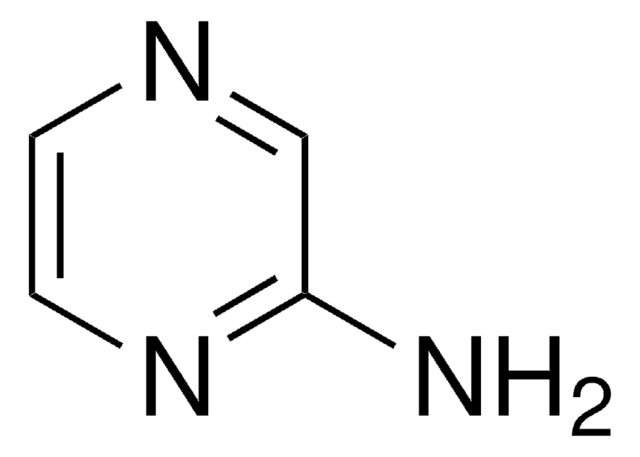
Empirical Formula (Hill Notation):
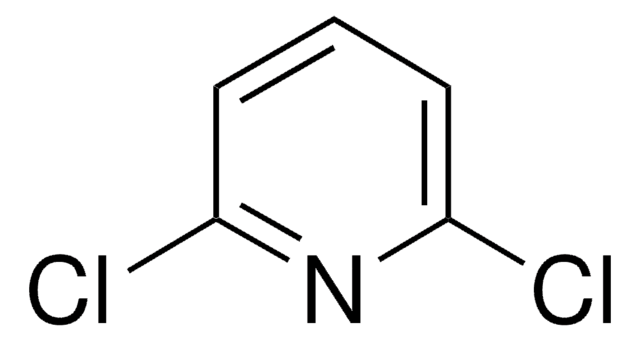
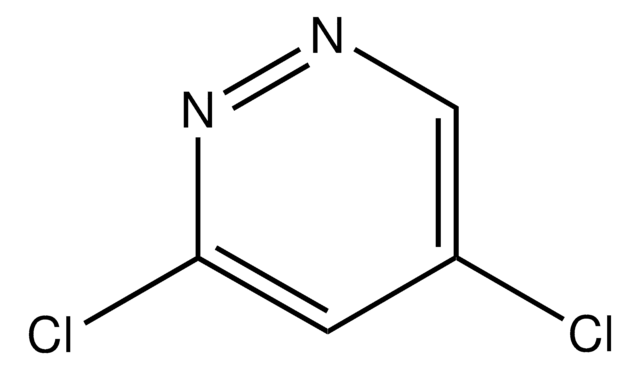
C4H2Cl2N2
CAS Number:
Molecular Weight:
148.98
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39162101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
55-58 °C (lit.)
SMILES string
Clc1cncc(Cl)n1
InChI
1S/C4H2Cl2N2/c5-3-1-7-2-4(6)8-3/h1-2H
InChI key
LSEAAPGIZCDEEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Laixing Hu et al.
Bioorganic & medicinal chemistry, 21(21), 6732-6741 (2013-09-10)
Dicationic 2,6-diphenylpyrazines, aza-analogues and prodrugs were synthesized; evaluated for DNA affinity, activity against Trypanosoma brucei rhodesiense (T. b. r.) and Plasmodium falciparum (P. f.) in vitro, efficacy in T. b. r. STIB900 acute and T. b. brucei GVR35 CNS mouse
Ling-Wei Kong et al.
Dalton transactions (Cambridge, England : 2003), 41(18), 5625-5633 (2012-03-17)
Cyclooligomerization of 2,6-dichloropyrazine 4 and benzyl 2,3-dihydroxybenzoate 5 under microwave irradiation resulted in a racemic pair of ester functionalized ortho-linked oxacalix[2]benzene[2]pyrazine 6, which was further transformed to the corresponding racemic carboxylic acid functionalized ortho-linked oxacalix[2]benzene[2]pyrazine 3. Both enantiomers of 3
Synthesis of oxacalixarenes incorporating nitrogen heterocycles: evidence for thermodynamic control.
Jeffrey L Katz et al.
Organic letters, 8(13), 2755-2758 (2006-06-16)
[structure: see text] Oxacalix[2]arene[2]hetarenes are formed in a single step by cyclooligomerization of meta-diphenols with meta-dichlorinated azaheterocycles. The high selectivity for cyclic tetramer formation results from thermodynamic product control. Macrocycles as large as oxacalix[5]arene[5]hetarenes have been isolated under nonequilibrating conditions.
Benjamin J Coe et al.
The Journal of organic chemistry, 75(24), 8550-8563 (2010-11-18)
Six new dicationic 2D nonlinear optical (NLO) chromophores with pyrazinyl-pyridinium electron acceptors have been synthesized by nucleophilic substitutions of 2,6-dichloropyrazine with pyridyl derivatives. These compounds have been characterized as their PF(6)(-) salts by using various techniques including electronic absorption spectroscopy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service