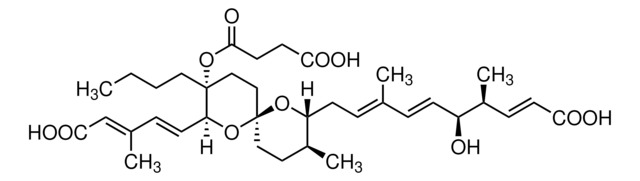
T6567
Trypsin from porcine pancreas
Proteomics Grade, BioReagent, Dimethylated
Synonym(s):
Porcine Trypsin, Trypsin for Mass Spectropetry
About This Item
Recommended Products
biological source
Porcine pancreas
Quality Level
product line
BioReagent
solubility
1 mM HCl: soluble 1 mg/mL, clear, colorless
shipped in
wet ice
storage temp.
2-8°C
Related Categories
General description
Application
- In-gel protein digestion and MALDI-TOF mass spectrometry analysis
- Electrospray Ionization Mass Spectrometry (ESI-MS) analysis
- Surface proteome profiling of L. plantarum
- Gel Filtration, Ultracentrifugation, and Rotary Shadowing Electron Microscopy
- Mass spectrometry
Biochem/physiol Actions
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Evaluation of Recombinant, Chemically Treated Trypsin in Proteomics and Protein Characterization Assays
The field of proteomics is continually looking for new ways to investigate protein dynamics within complex biological samples. Recently, many researchers have begun to use RNA interference (RNAi) as a method of manipulating protein levels within their samples, but the ability to accurately determine these protein amounts remains a challenge. Fortunately, over the past decade, the field of proteomics has witnessed significant advances in the area of mass spectrometry. These advances, both in instrumentation and methodology, are providing researchers with sensitive assays for both identification and quantification of proteins within complex samples. This discussion will highlight some of these methodologies, namely the use of Multiple Reaction Monitoring (MRM) and Protein-AQUA.
Protocols
This procedure is for products with a specification for Trypsin activity using Na-Benzoyl-L-arginine ethyl ester (BAEE) as a substrate. The procedure is a continuous spectrophotometric rate determination (A253, Light path = 1 cm).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service