BCR289
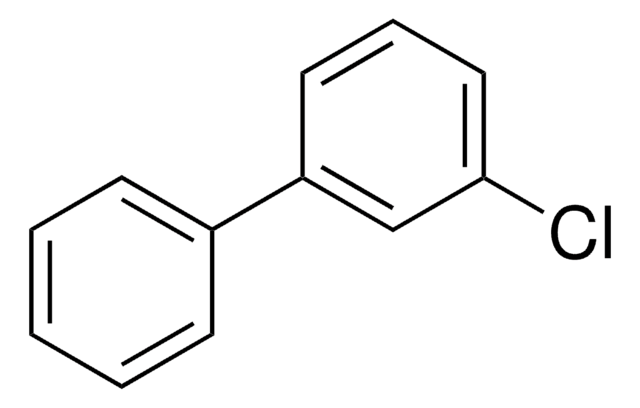
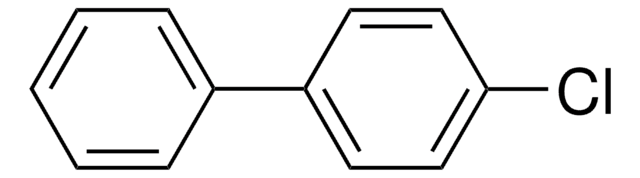
2,4′-Dichlorobiphenyl
BCR®, certified reference material
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H8Cl2
CAS Number:
Molecular Weight:
223.10
Beilstein:
1946417
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
certified reference material
Agency
BCR®
manufacturer/tradename
JRC
format
neat
storage temp.
2-8°C
SMILES string
Clc1ccc(cc1)-c2ccccc2Cl
InChI
1S/C12H8Cl2/c13-10-7-5-9(6-8-10)11-3-1-2-4-12(11)14/h1-8H
InChI key
UFNIBRDIUNVOMX-UHFFFAOYSA-N
General description
2,4′-Dichlorobiphenyl is a polychlorinated biphenyl (PCB) congener whose biphenyl nucleus is substituted with two chlorine atoms. PCBs are well-known environmental contaminants and are classified as persistent organic pollutants.
Application
2,4′-Dichlorobiphenyl may be used as a certified reference material (CRM) to quantify the analyte in environmental samples by chromatography techniques.(3)(4)
Analysis Note
For more information please see:
BCR289
BCR289
Legal Information
BCR is a registered trademark of European Commission
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wenjie Ren et al.
Environmental science and pollution research international, 25(20), 20084-20096 (2018-05-12)
Graphene may affect fate of organic contaminants due to its strong adsorption properties, which is very crucial for accurately assessing ecological risk of graphene and concurrent contaminants, while the current information remains largely unknown. Here, we firstly explored the adsorption
Robert M Burgess et al.
Environmental toxicology and chemistry, 23(11), 2534-2544 (2004-11-24)
Recent studies demonstrate that sedimentary black carbon (BC) affects the sorption of some hydrophobic organic contaminants (HOCs) to a greater extent than sedimentary organic carbon (OC). Among HOC, polycyclic aromatic hydrocarbons (PAHs) are known to interact extensively with BC. Currently
D L Busbee et al.
Archives of toxicology, 68(2), 96-102 (1994-01-01)
Absorption from the gastrointestinal tract and subsequent vascular transport of [3H]-2,4'-dichlorobiphenyl (Aroclor 1232; DCB) was investigated in an ovine model system. Rapid uptake of DCB and transport as a component of blood plasma without prior occurrence in thoracic duct lymph
Qi Lin et al.
Ying yong sheng tai xue bao = The journal of applied ecology, 16(4), 688-692 (2005-07-14)
To understand the surface chemistry of phenolic pollutants in the presence of metal oxides, this paper studied the reactions of 2,4-dichlorophenol with metal oxides in a kinetic and batch experiment. The results showed that amorphous ferric oxyhydroxide, goethite, delta-MnO2 and
Effect of three polychlorinated biphenyls on f-met-leu-phe-induced degranulation in rat neutrophils.
J Olivero-Verbel et al.
Toxicology letters, 98(3), 195-202 (1998-10-27)
It has been known that polychlorinated biphenyl (PCB) mixtures and individual congeners produce degranulation of rat neutrophils. Structure-activity relationships for congeners PCB 8 (2,4'-dichlorobiphenyl), PCB 126 (3,3',4,4',5-pentachlorobiphenyl) and PCB 128 (2,2',3,3',4,4'-hexachlorobiphenyl) were examined by correlating the extent of degranulation and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service