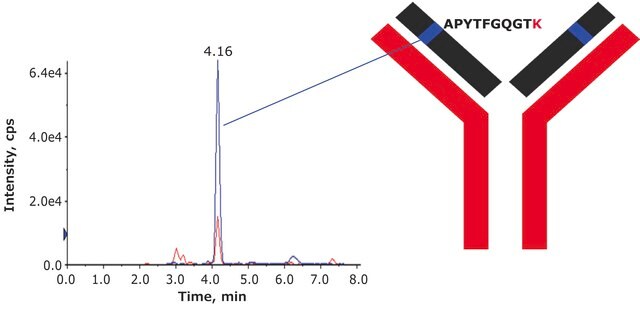
A-166
Adalimumab (Humira) solution
10.0 mg/mL (12.5 mM Histidine Buffer), certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 0.25 mL
manufacturer/tradename
Cerilliant®
concentration
10.0 mg/mL (12.5 mM Histidine Buffer)
technique(s)
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
General description
Application
Features and Benefits
- Adalimumab (Humira) content is quantitatively measured by amino acid analysis (AAA).
- Every raw material employed has been identified and comprehensively characterized using a variety of analytical methods.
- The fill volume is gravimetrically verified at various stages during the dispensing process using qualified and calibrated balances.
- Long-term stability has been evaluated under freezer storage conditions (-10 °C to -25 °C).
Preparation Note
- Thaw the product in a refrigerator or at room temperature and ensure thorough mixing before use.
- Avoid refreezing the product once thawed.
- When spiking into a matrix or diluting to required concentrations, it is recommended to quantitatively transfer the appropriate volume using established laboratory practices.
Other Notes
Legal Information
related product
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
A complete SEC-UV workflow for the characterization of mAb monomers, aggregates, and fragments using Zenix® and Zenix®-C SEC columns, including system suitability testing and forced pH and temperature stress studies.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service