DalPhos Ligands
Professor Mark Stradiotto and coworkers at Dalhousie University have developed air-stable Dalphos, which contains the bulky di(1-adamantyl)phosphino [P(1-Ad)2] fragment. These chelating N,P ligands are useful for previously unprecedented Pd-catalyzed C-N and C-C bond formation. Both Me-DalPhos (Cat. No. 751413) and Mor-DalPhos (Cat. No. 751618) allow for Pd-catalyzed ammonia cross-coupling. In particular, the more reactive Mor-Dalphos improved the scope and utility of ammonia coupling at room temperature.
Benefits of DalPhos Ligands:
- Monoarylation of ammonia, hydrazine and acetone
- Air-stable
- Good functional group tolerance
- Mild conditions
Me-DalPhos featuring an ortho-dimethylamino group has been shown to be a valuable ligand for the Pd-catalyzed cross-coupling of aryl chlorides and amines (including ammonia). The highly efficient Mor-Dalphos, where the dimethylamino group is replaced by morpholine, easily enables the ammonia coupling of a range of aryl chlorides and aryl tosylates.
Cross-Coupling of Ammonia

Mor-DalPhos can be used for the first Pd-catalyzed cross-coupling of aryl chlorides and tosylates with hydrazine. The Stradiotto group also explored the scope of the reaction of aryl hydrazines with benzaldehydes to afford the corresponding aryl hydrazones.
Cross-Coupling of Hydrazine

The direct arylation of acetone with a range of aryl halides and tosylates has been reported with good monoarylation selectivity. The use of Mor-DalPhos has a key impact on the rate and selectivity of this reaction.
Cross-Coupling of Acetone

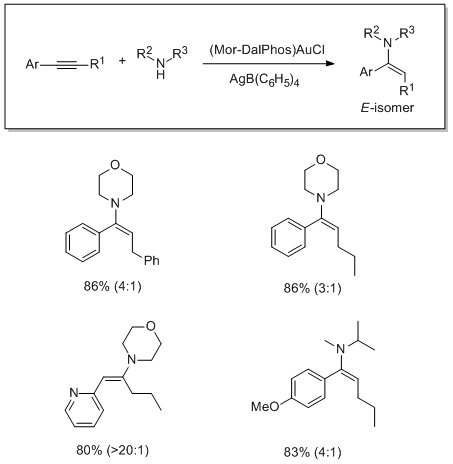
The use of Mor-DalPhos for the gold-catalyzed hydroamination of internal aryl alkynes with dialkylamines has been studied. This gold precatalyst featuring a P,N-ligand effects the stereoselective addition of a variety of functionalized dialkylamines to unsymmetrical internal arylacetylenes.
Gold-Catalyzed Hydroamination
Materials
To continue reading please sign in or create an account.
Don't Have An Account?

