554720
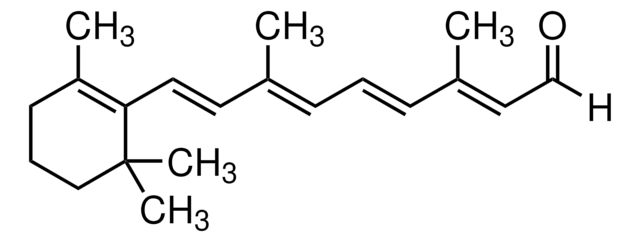
trans-Retinoic Acid
Potent modulator of growth and differentiation. Inhibits melanocyte adhesion, motility, and growth.
Synonym(s):
trans-Retinoic Acid, Tretinoin, ATRA, Vitamin A Acid
About This Item
Recommended Products
Quality Level
Assay
≥95% (by assay)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
color
yellow
solubility
DMSO: 25 mg/mL
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+
InChI key
SHGAZHPCJJPHSC-YCNIQYBTSA-N
General description
Application
- All-trans-retinoic acid modulates glycolysis via H19 and telomerase: the role of mir-let-7a in estrogen receptor-positive breast cancer cells.: This research reveals how all-trans-retinoic acid influences glycolysis in breast cancer cells by modulating H19 and telomerase, demonstrating its potential in breast cancer therapy (El Habre et al., 2024).
- Retinoic acid tiers mitochondrial metabolism to Sertoli Cell-Mediated efferocytosis via a non-RAR-dependent mechanism.: The study explores the role of retinoic acid in linking mitochondrial metabolism to efferocytosis in Sertoli cells, providing insights into its non-RAR-dependent mechanisms and potential applications in reproductive biology (Wu et al., 2024).
- Combined treatment of All-trans retinoic acid with Tamoxifen suppresses ovarian cancer.: This article discusses the synergistic effects of combining all-trans-retinoic acid with tamoxifen in suppressing ovarian cancer, highlighting a promising therapeutic strategy for ovarian cancer patients (Xu et al., 2024).
Packaging
Warning
Reconstitution
Other Notes
Clagett-Dame, M., et al. 1993. Arch. Biochem. Biophys.300, 684.
Labbaye, C., et al. 1993. Blood81, 475.
Sakashita, A., et al. 1993. Blood81, 1009.
Situ, R., et al. 1993. Dermatology186, 38.
Tini, M., et al. 1993. Genes Develop.7, 295.
Leid, M., et al. 1992. Trends Biochem. Sci.17, 427.
Sharpe, C.R. 1991. Neuron7, 239.
Thaller, C. and Eichele, G. 1990. Nature345, 815.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service