901776
Poly(OEGMA)
hydrazide functionalized, 25 wt% solution in water
Synonym(s):
PEGMA, POEGMA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
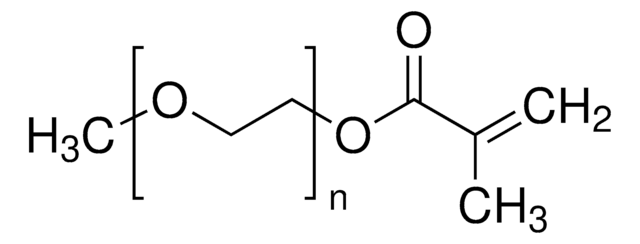
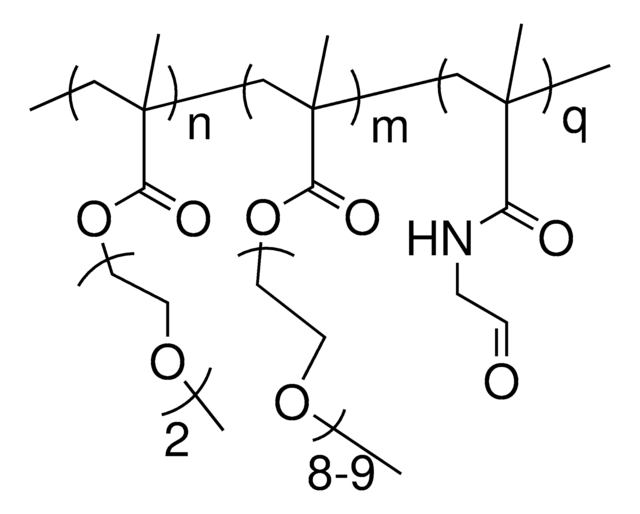
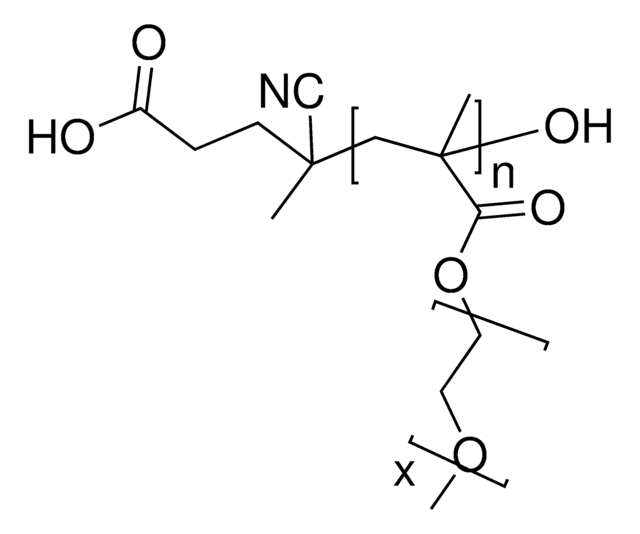
[CH2CCH3(COO(CH2CH2O)8CH3)]m[CH2CHCONHNHCO(CH2)4CONHNH2]p
UNSPSC Code:
12162002
NACRES:
NA.23
Recommended Products
form
solution
mol wt
~20,000 g/mol
color
clear colorless to pale yellow
functional group
hydrazide
storage temp.
2-8°C
General description
Poly(oligoethylene glycol methacrylate) (POEGMA), is a comb-shaped, graft copolymer consisting of hydrophilic oligomer PEG chains grafted to a hydrophobic polymethacrylate backbone. POEGMA has been suggested as a viable alternative to poly (ethylene glycol) (PEG) in biological and biomaterial applications. POEGMA has been reported to improve pharmacokinetic properties of protein and peptide conjugates, enhance the stability and gene silencing efficiency of siRNAs, as an anti-fouling surface for biosensors, and eliminate PEG antigenicity. In addition to use in biomolecule-polymer conjugates, PEOGMA has also seen wide spread use in tissue engineering applications, such as hydrogel synthesis. Hydrazide-functionalized POEGMA can be readily used with the corresponding aldehyde-functionalized POEGMA for rapid gelation via reversible hydrazone bond formation. Due to the reversibility of the bond formation and the low viscosity of the precursors, resulting hydrogels can be used as injectable tissue engineering matrices, local drug delivery vehicles for small molecules, or as joint lubricants. In addition, the physical properties of the resulting hydrogels, such as LCST, gelation rates, swelling kinetics, degradation kinetics, and mechanical properties, can all be readily controlled by solution concentration and the ratios of each solution.
Preparation Note
This product is provided as a 25 wt% solution in water, ready to be diluted for your specific application. Please see the technical bulletin on the product page for dilution and hydrogel preparation instructions.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactive electro spinning of degradable poly(oligoethylene glycol methacrylate)-based nanofibrous hydrogel networks.
Xu F, et al.
Chemical Communications (Cambridge, England), 52, 1451-1454 (2016)
?Off-the-shelf? thermoresponsive hydrogel design: tuning hydrogel properties by mixing precursor polymers with different lower-critical solution temperatures.
Bakaic E, et al.
Royal Society of Chemistry Advances, 5, 33364-33376 (2015)
A. brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity.
Qi Y, et al.
Nmr in Biomedicine, 1, 0002-0002 (2016)
A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity.
Qi Y, et al.
Nature Biomedical Engineering, 1, 2-2 (2016)
Imran Ozer et al.
Biomacromolecules, 18(9), 2699-2710 (2017-08-05)
PEGylation, covalent attachment of PEG to therapeutic biomolecules, in which suboptimal pharmacokinetic profiles limiting their therapeutic utility are of concern, is a widely applied technology. However, this technology has been challenged by reduced bioactivity of biomolecules upon PEGylation and immunogenicity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service