Famciclovir Tablets USP Monograph Method Using a Purospher™ STAR RP-8 endcapped HPLC Column and UV Detection
Sonal Shinde, Application Specialist
Famciclovir is an antiviral drug indicated for the treatment of herpes zoster, herpes simplex virus 2 (genital herpes), herpes labialis (cold sores), etc. It is a guanosine analogue, a prodrug form of penciclovir, and marketed by Novartis under the trade name Famvir. Generics are produced by TEVA and Mylan, among others. Purospher™ STAR RP-8 endcapped HPLC columns can be used to monitor organic impurities in tablet formulations following the new USP monograph for Famciclovir Tablets. The method suitability requirements are defined by the relative standard deviation (NMT 5.0% for Famciclovir standard solution) and the chromatographic resolution between propionyl famciclovir and 6-chloro famciclovir (NLT 1.2 using the system suitability solution).
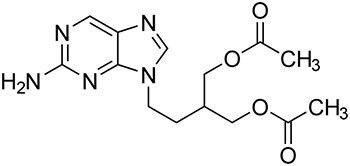
The method acceptance criteria are defined by the relative retention times for Famciclovir related compound A, Famciclovir related compound B, Famciclovir, 6-Chloro famciclovir, and Propionyl famciclovir and are about 0.2, 0.5, 1.0, 1.32, and 1.35, respectively. This application note illustrates with required analytical data that the method meets USP41-NF36 guidelines.
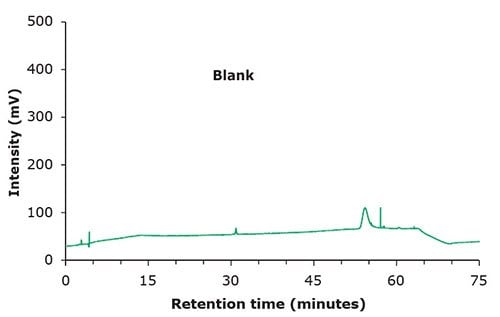
Figure 1. Chromatographic data – blank & standard solution.
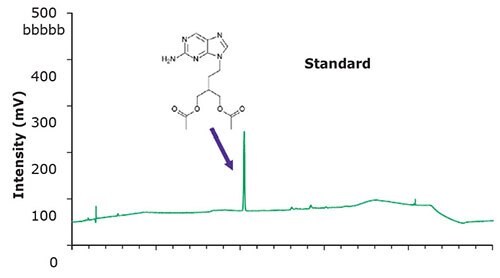
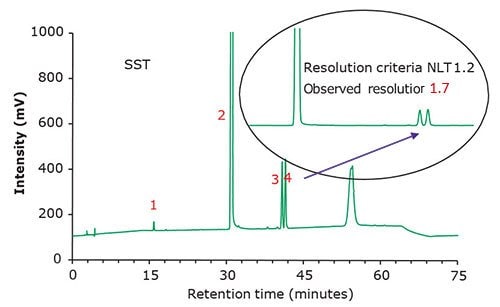
Figure 2.Chromatographic data - system suitability test (SST) solution.
Figure 3. Chromatographic data - test solution.
Validation and verification
1. Specificity
Inject solution and determine the retention time of desired analyte in the presence of other components such as impurities and excipients.
2. Standard Repeatability (1 ppm)
3. Linearity, LOD & LOQ
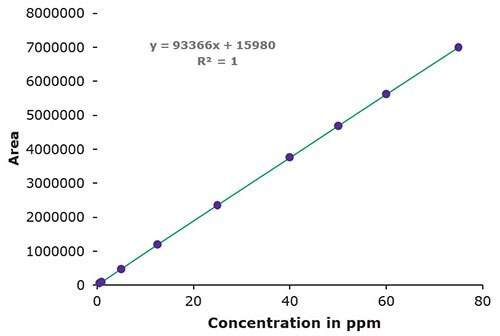
Figure 4.Area / Concentration in ppm
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?