Sensitivity of Lateral Flow Diagnostic Assays with Ultra-Bright Gold Nanoshell Reporters
Introduction
Lateral flow assays (also known as immunochromatographic assays) detect or quantify biomolecules in complex samples including blood, urine, saliva, or other fluids to diagnose a variety of medical conditions.1 They are usually self-contained, portable devices that are easy to use, fast, and inexpensive. Lateral flow test devices can be stored at ambient temperature, have a long shelf life, and provide diagnostic results without complex sample processing or additional equipment, making them ideal for both point-of-care and field-based diagnostic uses. Hundreds of millions of lateral flow tests are used each year for a wide variety of applications, including the ubiquitous pregnancy test (Figure 1). The sensitivity of these assays depends on the components used, how the components and sample are treated, and the properties of the nanoscale reporter particles that generate the diagnostic signal. The reporter particles are labeled with a molecule — often an antibody or nucleic acid — that recognizes the analyte in the sample and binds to a specific physical location on the strip. To maximize the sensitivity of the assay, reporters are chosen to be as “bright” as possible, generating the largest possible signal intensity per binding event. The reporter particle must also be very small, typically on the nanoscale, so that it can effectively bind to molecular targets.
Lateral flow assays consist of a series of inexpensive, paper-like components that are assembled onto a backing card for handling (Figure 2). Components of lateral flow strips typically include:
- Sample pad: Absorbs sample and controls distribution and flow of sample onto the conjugate pad
- Conjugate pad: Dispenses and dries nanoparticle-antibody conjugates, ensuring controlled release of conjugate onto the nitrocellulose membrane upon hydration
- Nitrocellulose membrane: Immobilizes the Test line and Control line reagents
- Wicking pad: Provides uniform capillary flow through the membrane, absorbs applied sample, and prevents backflow

Figure 1.Image of commonly used lateral flow rapid test for determining pregnancy.
Each component overlaps the previous one by at least 1 mm, which allows the sample to flow unimpeded from the sample pad all the way to the wicking pad.

Figure 2.Schematic of lateral flow test strip.
To use a lateral flow rapid test, a liquid sample such as blood, serum, plasma, urine, saliva, or solubilized solids is placed on the sample pad; capillary action then draws the sample through the lateral flow device. The sample pad is sometimes treated with reagents to adjust the pH of the sample, or can contain filters to remove unwanted particulates such as red blood cells. The sample flows from the sample pad to the conjugate pad, which contains dried, strongly colored or fluorescent nanoparticles with antibodies conjugated to their surface. When the sample reaches the conjugate pad, the nanoparticles are rehydrated and mixed with the sample. The mixture then flows through the nitrocellulose membrane and across one or more test lines and a control line. The test and control lines are comprised of immobilized proteins that interact with the sample and generate a signal. The strength of the signal corresponds to the amount of analyte or conjugate present. The remaining fluid is absorbed by a wicking pad that is designed to capture the sample and prevent backflow. Once all the sample has finished running, the signal contrast at the test line(s) can be read by either visual interpretation or using a reader to identify the presence of the analyte, and thus the results.
Lateral flow assay formats
The two most common lateral flow assay formats are “sandwich” and “competition” (Figure 3). The sandwich assay format is typically used for detecting larger analytes that have at least two epitopes (binding sites). In most cases, an antibody to one epitope is conjugated to the nanoparticle (the detection antibody on the reporter particle), and an antibody to another epitope is used for the assay’s test line (capture antibody). If there is analyte in the sample, it binds to both the detection and capture antibodies, creating a sandwich between the nanoparticle conjugate and the test line to produce a positive signal. If no analyte is present, the nanoparticle conjugate doesn’t bind to the capture antibody and no signal is observed. Sandwich assays result in a signal intensity on the test line that is directly proportional to the amount of analyte present in the sample.
The competition assay format is used to detect analytes when antibody pairs are unavailable, or if the analyte is too small for multiple antibody binding events, such as steroids or small organic molecule drugs. In this format, the test line typically contains the analyte molecule, usually a protein-analyte complex. If the target analyte is present in the sample, it binds to the reporter particle and prevents it from binding to the analyte embedded in the test line. If no analyte is present, the reporter particles bind to the analyte embedded in the test line, yielding a signal. In the competition format, signal intensity is inversely proportional to the amount of analyte present in the sample.
In both assay formats, an anti-species antibody is used as the control line, binding the conjugated antibody regardless of test line result. Presence of a control line confirms proper flow and functionality of the assay.

Figure 3.Depiction of “Sandwich” and “Competition” assay formats. In a sandwich assay, a positive signal indicates the presence of analyte and the test line intensity is proportional to the amount of analyte available in the sample. For a competition assay, a strong signal on the test line means little or no analyte is present. This signal intensity is inversely proportional to the analyte concentration.
Nanoparticles as reporters in lateral flow
Lateral flow tests generate an optical signal from strongly colored or fluorescent particles that are bound to test lines on a white nitrocellulose strip. This signal can be read by eye (qualitative or semi-quantitative) or by an optical reader (quantitative). To maximize the sensitivity of the test, each binding event between an analyte and reporter particle should produce the strongest signal possible. While larger particles typically provide a stronger signal per binding event, particles that are too large don’t easily flow through the nitrocellulose membrane and have limited opportunity to bind to the test line. Thus, particles that are between 20 nm and 500 nm in diameter are typically selected for use in lateral flow assays. Fluorescent particles can also be employed, and the same general rules apply: the more potent the fluorescence per binding event, the stronger the signal.
One of the most common reporter particles used in diagnostic assays are gold nanoparticles, often referred to as gold colloid.2 Gold nanoparticles have unusual optical properties that make them exceptionally strong absorbers of light. For example, 40 nm diameter gold nanospheres strongly absorb light at wavelengths near 520 nm, resulting in a ruby red test line. The gold surface has a natural affinity for antibodies and other proteins, allowing fabrication of nanoparticle-antibody conjugates by simply mixing gold nanoparticles with an affinity ligand. Other sizes and shapes of gold nanoparticles have also been used as lateral flow probes. Gold nanoshells with a 150 nm diameter provide a higher contrast per binding event — typically providing a 5–20 fold increase in sensitivity as compared to 40 nm gold nanospheres. Gold nanoshells are prepared by the electroless deposition of metallic gold onto the surface of a silica particle that is studded with small gold nanoparticles.3 The small gold seeds grow progressively larger, until they coalesce into a complete shell. (Figure 4)

Figure 4.Transmission electron microscopy images of different stages of nanoshell growth. Initially, thousands of ~2 nm gold nanoparticle seeds are bound to positively charged silica nanospheres (left). Gold ions in solution are electrolessly deposited onto the bound gold seed, growing the seeds larger (middle) until they coalesce into a complete shell (right).
By controlling the size of the silica core and the thickness of the gold shell during gold nanoshell synthesis, the peak wavelength of the nanoshells can be tuned to change the particle’s color. To maximize sensitivity in lateral flow assays, blue-gray 150 nm gold nanoshells with a 120 nm diameter silica core and a ~15 nm gold shell have been developed. The silica core has a much lower mass than gold, improving the settling time and flow characteristics in the nitrocellulose membrane relative to solid gold particles. Figure 5 compares the optical extinction (scattering + absorption) per particle of 40 nm gold nanospheres and 150 nm nanoshells. Because of their stronger extinction per particle, each nanoshell binding event exhibits a much higher contrast against the nitrocellulose substrate, resulting in increased assay sensitivity.

Figure 5.Per particle optical extinction (optical density per particle) as a function of wavelength for 40 nm gold nanospheres and 150 gold nanoshells.
Figure 6 shows test and control lines using gold nanoshell reporters, with a corresponding scanning electron microscope image. Individual nanoshells bound to antibodies and then immobilized on the nitrocellulose membrane can be seen as light-colored spheres on the darker nitrocellulose fibers. Even at the relatively low coverage density on the membrane, the control line appears very dark, demonstrating that each nanoshell binding event generates a high contrast signal against the white nitrocellulose background.

Figure 6.Gold nanoshells bound to a nitrocellulose membrane.
Ultimately, the sensitivity of the assay is determined by the number of reporters that must bind to see a visible test line. To measure the number of particles required to obtain a visual signal, streptavidin-coated 40 nm gold nanospheres and streptavidin-coated 150 nm gold nanoshells were captured on a biontinylated test line and the signal was analyzed with an optical reader (Figure 7). With 40 nm gold nanospheres there was a visible test line when 5 million particles were added to the assay. With gold nanoshells, only 500,000 particles were required to see a line, an order of magnitude decrease that yields increased assay sensitivity.
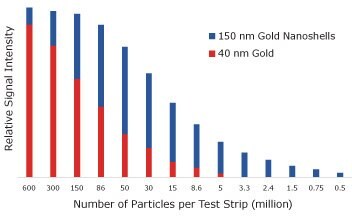
Figure 7.Lateral flow assay results where streptavidin-functionalized 150 nm gold nanoshells and 40 nm gold nanoparticles were placed on a nitrocellulose strip and captured on a biotinylated bovine serum albumin test line. The visual detection limit of gold nanospheres is 5,000,000, but for nanoshells it is 500,000 binding events.
To see how the absorbance per particle affects assay sensitivity, 40 nm diameter gold nanoparticles and 150 nm diameter gold nanoshells were used to detect Troponin I, a cardiac marker, in a lateral flow sandwich assay (Figure 8). The limit of detection with 40 nm gold nanoparticles was 0.5 ng/mL, while Troponin I detection with 150 nm gold nanoshells was an order of magnitude better at 0.05 ng/mL.
Covalent linkage chemistry for high stability reporters
Another important factor when developing high-sensitivity lateral flow assays is the method of binding antibodies or other proteins to the nanoparticle surface. Robust and effective binding is critical for maximizing both sensitivity and selectivity of the assay. The process of binding antibodies to the surface of a reporter particle is conjugation, and the resulting antibody-coated particle is called a conjugate.4,5 Passive adsorption (physisorption) is the traditional method for attaching proteins to lateral flow nanoparticle probes, and is still widely used. By taking advantage of the forces between molecules and surfaces at a specific pH (e.g. van der Waals and ionic forces), antibodies can be made to spontaneously bind to a bare gold nanoparticle surface to form a conjugate. The antibody is typically added in excess, to ensure the entire surface of the nanoparticle is covered. Any antibody remaining free in solution after the conjugation is complete is removed via centrifugation or filtration.

Figure 8.Sensitivity of lateral flow assay for Troponin I using 150 nm gold nanoshells and 40 nm gold nanospheres as reporter particles.
Conjugates can also be formed by covalently binding the antibody to the nanoparticle surface. Covalent binding is typically more reproducible than physisorption and for some antibodies, covalent chemistry is necessary to prepare stable conjugates. Covalent binding chemistry also allows for more control over the amount of antibody on the surface of the nanoparticles and the antibody orientation.
Other advantages of covalent binding include:
- Less antibody is needed to maximize sensitivity, reducing overall cost
- Covalent conjugates exhibit greater stability, allowing their use in difficult sample matrices and high salt/detergent buffering environments
- Conjugates are easily prepared without requiring extensive salt or pH optimizations, saving time when performing antibody screening experiments
- The antibody-to-particle ratio can be precisely controlled, which is important for adjusting the dynamic range in competitive assays and optimizing sensitivity with antibodies with varying binding kinetics
One common method of fabricating covalent conjugates utilizes carboxyl (carboxylic acid) functionalized gold surfaces. A carboxylic acid on the nanoparticle can be linked to a primary amine in the lysine residues of the antibody or protein using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysulfosuccinimide (sulfo-NHS) reagents to form amide bonds. A typical IgG antibody will have 80–100 lysine residues, of which 30–40 will be accessible for EDC/NHS binding.
Interpreting results from lateral flow tests
The results from a lateral flow test can be qualitative (if an analyte is or isn’t present within the limits of detection), semiquantitative (analyte present at low, medium, or high levels) or quantitative (precise amount of analyte present determined). The ubiquitous pregnancy test is an example of a qualitative “yes”/”no” assay, where a positive test line signal correlates to elevated levels of the hCG hormone in urine, indicating that the user is pregnant. For quantitative diagnostics, the test line intensities are compared to a calibration standard and converted to an analyte concentration value. Quantitative assays are used to measure the concentration of a specific analyte or biomarker, instead of simply indicating the presence or absence. For example, someone who experiences high stress may want to accurately measure the concentration of their cortisol levels over time, to determine if stress mitigation interventions are working. To accurately measure the test line intensity, the result must be analyzed by a strip reader. The recent commercialization of readers with a small form factor that are also inexpensive and mobile-centric is transforming the lateral flow assay industry. Simple methods that can quantify the output of lateral flow assays without requiring a stand-alone bench top reader opens a tremendous opportunity for home use and point-of-care diagnostics. Figure 9 shows a variety of currently available disposable and benchtop reader formats. For benchtop units, a lateral flow cartridge is inserted into the instrument, the test and control line intensities are imaged with a camera, and the results are sent to a computer or mobile phone for display and interpretation. The calibration curve for any given assay can be encoded into the software, such that the test line signal intensity can be automatically converted to analyte concentration and presented to the user. Disposable readers have all of the necessary hardware to perform the analysis on the device itself, further simplifying the user experience.

Figure 9.Various Lumos Diagnostic reader formats for lateral flow diagnostics.
Conclusions
Ultra-bright reporter particles based on the unique optical properties of gold nanoshells significantly increase the sensitivity of lateral flow immunoassays. When combined with recent developments in portable reader technology, a new class of point-of-care diagnostics are coming to market that have the sensitivity, specificity, and reproducibility of laboratory-based tests but in a much less expensive and more convenient test format. In addition to lateral flow assays, gold nanoshells can be used in a wide variety of diagnostic and detection applications including surface enhanced Raman spectroscopy, flow cytometry, and molecular imaging. With simple covalent coupling chemistry and easy to follow protocols, gold nanoparticle conjugates are an important tool for many biotechnology applications.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?