Application of Carbon Monoxide in Synthesis Made Simple and Safe by Prof. Skrydstrup and Coworkers
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.1 The solid and bench stable CO-precursor, COgen, was found to be a highly efficient and reliable source of gaseous CO. Palladium mediated CO-release from COgen in near quantitative amounts occurs in any aprotic solvent and at a wide temperature range. Furthermore, its carbon-13 labeled counterpart, 13COgen, provides the corresponding 13C-carbon monoxide under identical conditions.
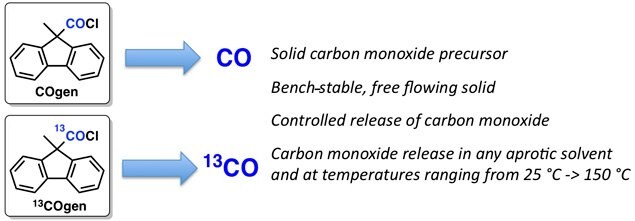
Figure 1.Application of Carbon Monoxide in Synthesis Made Simple and Safe by Prof. Skrydstrup and Coworkers
The combination of COgen or 13COgen with the two-chamber system, COware, is a safe and simple method for performing carbonylation chemistry. Release of CO in one chamber and its direct application in the secondary chamber provides a method in which the direct handling of CO is avoided. Substitution of 13COgen for COgen yields the labeled product under identical conditions.1 This versatile method is now available through Sigma Aldrich as laboratory hardware and bench-stable CO precursors.
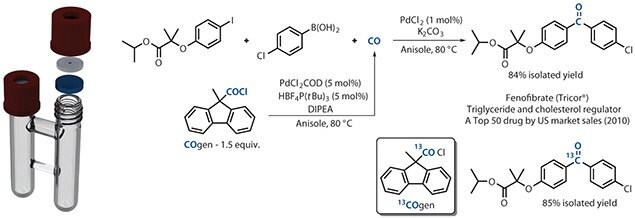
Figure 2.The combination of COgen or COgen with the two-chamber system
To date, this CO technique has found application in numerous carbonylation purposes including the classical amino-,1,2 alkoxy-,3
thio-4 and reductive carbonylations.5 Furthermore, new conditions were developed providing access to carbonylative versions of the Suzuki-Miyaura coupling,6 the Mizoroki-Heck reaction,7 the α-arylation8 and even access to α-ketoamides6 by a double carbonylation was obtained by the COware-COgen combination.9,10 Again, the carbon-13 labeled compounds were obtained by simple substitution of 13COgen for COgen.
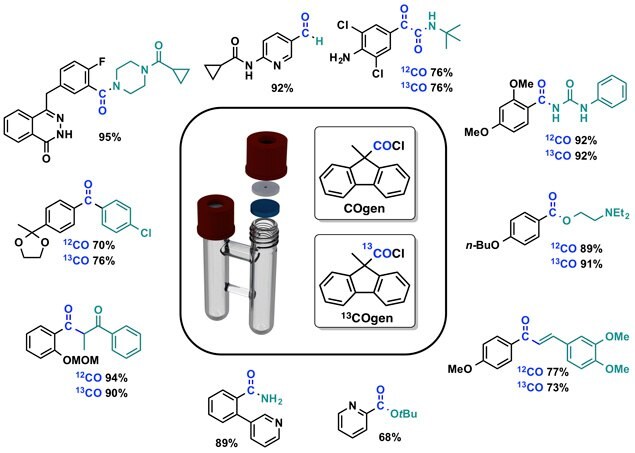
Figure 3.COgen
CO generated in the COware system is directly comparable to CO delivered from a pressurized canister and does not require setup in a glove box. COware comes with PTFE-stabilizing discs, which ensures the PTFE-silicone seal is resealable. The method fits into any fume hood and allows simple and safe protocols for carrying out chemistry applying CO.
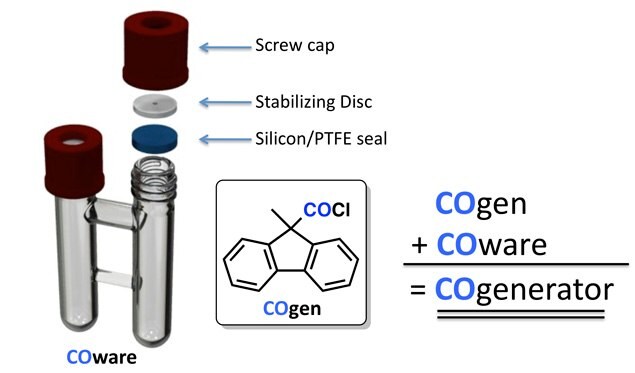
Figure 4.CO gen in the COware system is directly comparable to CO
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?