What is Copper-Free Click Chemistry?
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.1 The absence of exogenous metal catalysts makes these reactions suitable for the in vivo applications of bioorthogonal chemistry or bioorthogonal click chemistry.
Wittig’s Copper-Free Click Reaction
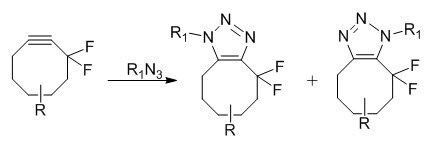
Copper-free click chemistry is based on a very old reaction, published in 1961 by Wittig et al. It involved the reaction between cyclooctyne and phenyl azide, which proceeded like an explosion to give a single product, 1-phenyl-4,5,6,7,8,9-hexahydro-1H-cycloocta[d][1,2,3]triazole.2 The reaction is ultrafast due to the large amount of ring-strain (18 kcal/mol of ring strain) in the cyclooctyne molecule. Release of the ring-strain in the molecule drives the fast reaction. Cyclooctynes are reported to react selectively with azides to form regioisomeric mixtures of triazoles at ambient temperatures and pressures without the need for metal catalysis and no apparent cytotoxicity.3 Difluorinated cyclooctyne reagents have been reported to be useful for the copper-free click chemistry4
Copper-Free Click Chemistry Applications
- Labeling of biomolecules, such as glycans and lipids, selectively in living systems with no apparent toxicity.3
- Labeling biomolecules in live mice, by a bioorthogonal reaction, the 1,3-dipolar cycloaddition of azides and cyclooctynes.5
- Biarylazacyclooctynone (BARAC) can be readily synthesized and employed for live cell fluorescence imaging of azide-labeled glycans.6
- In situ “click” cross-linking of azide-terminated photodegradable star polymers, by employing bifunctional, fluorinated cyclooctynes.7
- Functionalization of new platinum(IV) [PtIV] prodrugs. These prodrugs may be utilized for the installation of targeting moieties, delivery systems and fluorescent reporters from a single precursor that are capable of releasing biologically active cisplatin.8
- Functionalization of gold nanoparticles with monovalent maleimide, by the installation of a maleimide group on nanoparticle via copper-free click chemistry.9
- Synthesis of biocompatible and biodegradable polysaccharide hydrogels derived from chitosan and hyaluronan have been achieved by copper-free click chemistry. These hydrogels have potential soft-tissue engineering applications.10
- Triazole analogs of phthalate plasticizers (PVC-DEHT, PVC-DBT and PVC-DMT) have been prepared by copper-free azide-alkyne click reaction. Di(2-ethylhexyl)-1H-triazole-4,5-dicarboxylate (DEHT), di(n-butyl)-1H-1,2,3-triazole-4,5-dicarboxylate (DBT), and di(methyl)-1H-triazole-4,5-dicarboxylate (DMT) are covalently attached to azide-functionalized polyvinyl chloride (PVC) via copper free-click reaction.11
Scheme of the above mentioned syntheses:

- Novel class of difluorinated cyclooctyne (DIFO) reagents were employed in copper-free click chemistry for the site-selective labeling of biomolecules in vitro and in vivo.12
- Catalyst-free click reactions are useful tools for the preparation of radiometal-based pharmaceuticals. Radiotracer [64Cu]DOTA-ADIBON3-Ala-PEG28-A20FMDV2, used for positron emission tomography imaging of integrin αvβ6 expressing tumors, has been synthesized via copper-free click chemistry.13
- Iodine radioisotope labeling of cyclooctyne-containing molecules by copper-free click reaction has been reported. Radioiodination using the tin precursor was carried out at room temperature to obtain 125I-labeled azide. Dibenzocyclooctyne (DBCO) containing cRGD peptide and gold nanoparticle were labeled by employing 125I-labeled azide to afford triazoles in good radiochemical yields (67–95%). This method is useful for both in vitro and in vivo labeling of DBCO group containing molecules with iodine radioisotopes.14
- Protein site-specific labeling techniques involve copper-free strain-promoted azide–alkyne cycloaddition (SPAAC) reaction between dibenzocyclooctyne-fluor 545 (DBCO-fluor 545) and an azide-bearing unnatural amino acid (UAA).15
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?