Endothelial Cell Tube Formation Angiogenesis Assay
What is Angiogenesis?
The formation of new blood vessels in the body are generated by three processes: vasculogenesis, arteriogenesis and angiogenesis.
- Vasculogenesis only occurs at an early stage of development, giving rise to the primitive circulatory system, while angio- and arteriogenesis (also) take place in adulthood.
- Arteriogenesis involves the remodeling and maturation of existing vessels to yield fully developed, functional arteries, usually when larger arteries are occluded.
- Angiogenesis is the physiological formation of new blood vessels from existing ones, a process that is essential for embryonic and fetal development and organ growth, supports the healing of wounds and skeletal growth, and is also an integral part of pregnancy and the female reproductive cycle.1, 2 It is triggered by tissue hypoxia or insufficient oxygen tension.3 Newly formed blood vessels lined with endothelial cells supply oxygen and nutrients to tissues, promote immune surveillance by hematopoietic cells, and remove waste products.2
Angiogenesis is a tightly regulated process that is balanced by pro- and antiangiogenic signals including integrins, chemokines, angiopoietins, oxygen sensing agents, junctional molecules and endogenous inhibitors.4 It is a hallmark of over 50 different disease states, and its dysfunction is implicated in cancer, psoriasis, various eye diseases, rheumatoid arthritis, asthma and other autoimmune diseases, infectious diseases, coronary arterial diseases, stroke, atherosclerosis and impaired wound healing, among others.1, 5
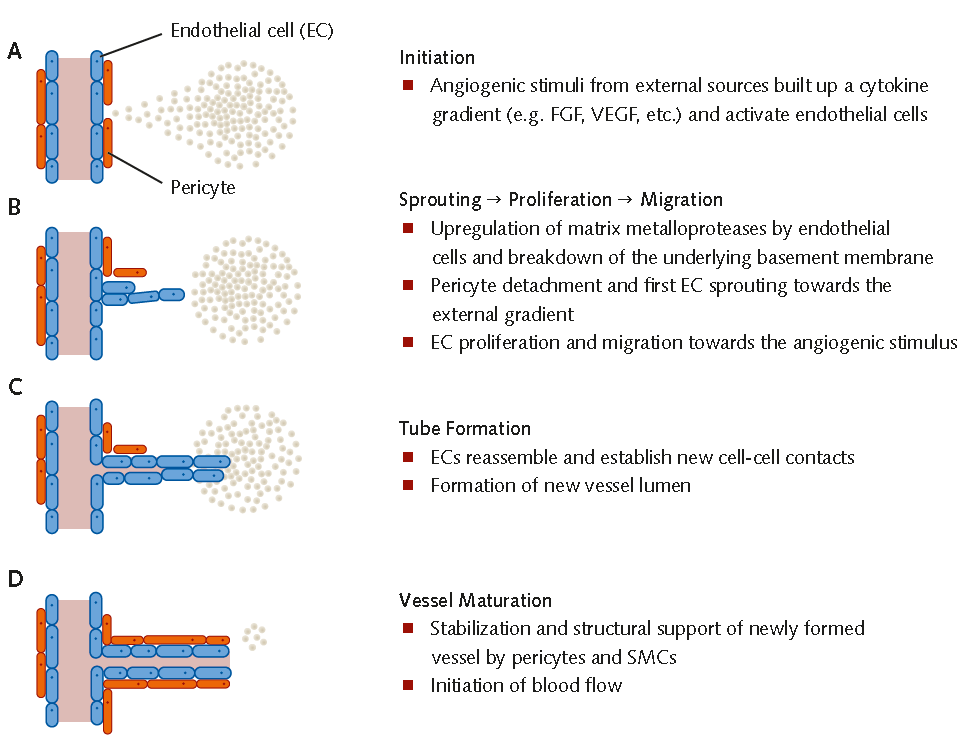
Figure 1. Mechanisms of Angiogenesis(A) Angiogenic stimuli give rise to a cytokine gradient and activate endothelial cells. (B) Endothelial cells break down the basement membrane, sprout toward the external gradient and begin to migrate toward the angiogenic stimulus. Pericytes detach from the blood vessel. (C) Reassembly of endothelial cells and formation of new cell-cell contacts and vessel lumina. (D) Vessel stabilization by pericyte recruitment and initiation of blood flow.
Assays to Study Angiogenesis
The cell culture tube formation assay first described in 1988 by Kubota et al.7 is one of the most widely used in vitro assays for angiogenesis. The assay involves plating endothelial cells onto a basement-membrane-like substrate on which the cells form tubules within six to 20 hours. These tubules mostly contain a lumen, and the cells develop tight cell-cell and cell matrix contacts. Quantification can be performed by measuring the tube area or the length and/or number of branch points, with area measurement being the most common and easiest method.6; 10 This powerful semiquantitative assay has many advantages. It is quick and easy to perform, can be scaled up for high-throughput analysis, and factors can be added exogenously to the medium, transfected into the cells, or knocked down.9
The Role of Basement Membrane in Angiogenesis
The basement membrane is an important extracellular matrix found in all endothelial tissues. It maintains tissue integrity, serves as a barrier between cells and proteins, separates different tissue types, transduces mechanical signals, has many biological functions that help maintain tissue specificity, and acts as a storage depot for growth factors and enzymes. Its major components are laminins, collagen IV, heparan sulfate proteoglycans, various growth factors, cytokines, chemokines and proteases. For the tube formation assay, basement membrane can be prepared from tissues or tumors, 11 or else a gel matrix layer of fibrin, collagen or Matrigel/ECM Gel can be used. The type of matrix used is important, as different matrices result in different rates of differentiation. It appears that the mechanisms by which endothelial cells differentiate to form tubes are at least partly dependent upon the matrix onto which the cells are plated; it is therefore advisable to test substances on more than one matrix type.6
Choosing the Correct Endothelial Cell Type
A crucial aspect for ensuring successful experiments is the right choice of endothelial cells. The tube-forming capacity varies among different groups of endothelial cells, making it essential to choose the assay conditions and cell types that most closely resemble the angiogenic conditions or disease being studied. In adult humans, there is a high degreeof heterogeneity among endothelial cells along the vascular tree. This facilitates biological adaptation to local requirements. Functional heterogeneity among endothelial cells is involved in controlling vasoconstriction and vasodilation, blood coagulation, fibrinolysis, antigen presentation, atherogenesis, and catabolism of lipoproteins. There is also considerable heterogeneity in endothelial cells derived from different locations within the body, related to the microenvironmental conditions in each organ and the specialized roles that the endothelium plays in them.6
PromoCell supplies a wide variety of endothelial cells from dermal, cardiac, pulmonary, uterine and saphenous tissuesas well as from large vessels and umbilical cords. Theoretically, all these endothelial cells ought to be able to build tubes under the right conditions. However, the extent of tube formation and their responses to angiogenesis stimulators and inhibitors vary greatly depending on the cell type. The tube formation assay presented in the following section has been tested for HUVEC, HDMEC, HCAEC, HPMEC, HAoEC and HCMEC in particular.
Endothelial Cell Tube Formation Assay Protocol
- Seed endothelial cells and allow them to grow. Plate endothelial cells in an appropriate culture vessel using the recommended PromoCell growth medium. Use seeding densities between 5X103 cells/cm2 and 2X104 cells/cm2 as recommended in the respective product manual. Replace culture medium every 2–3 days. Allow the cells to reach 70 – 90% confluency.
- Thaw basement membrane extract and prepare 96-well plate. Remove Basement Membrane Extract-BME (such as Matrigel or ECM Gel) from the freezer and place it in a refrigerator on ice. Thawing process will be completed after overnight-incubation at 4 °C. Label wells of the 96-well plate according to your experimental approach and pre-cool it in the refrigerator overnight.
- Adjust media and reagents to room temperature. Place PBS (D8537), Accutase (A6964), recommended Endothelial Cell Basal Medium and recommended growth medium at room temperature for at least 1 hour.
- Coat 96-well plate with Basement Membrane Extract. Place a tube of fully thawed BME on ice. Invert the tube for a few times. Load 50-80 μL of BME per well of the pre-cooled 96-well plate. BME should be evenly distributed across each well. Incubate the 96-well plate in a humidified incubator (37 °C, 5% CO2) for 30 min–1 hour. Proceed with step 5–8 during this incubation process.
- Prepare test media. Dissolve test substances in recommended Basal Medium. 1 mL of each test medium is needed to resuspend 1X105 – 1.5X105 endothelial cells.
- Detach endothelial cells. Endothelial Cells should be 70 – 90% confluent. Remove medium from the culture vessel and wash the cells twice with PBS (D8537). Remove the washing solution and add 50 μL Accutase (A6964) solution per cm2 of vessel surface. Close the vessel and incubate at 37 °C for 3–5 min. Examine cells under a microscope. Gently tap the side of the vessel to accelerate cell detachment. When about 80% of the cells have detached, add 100 μL of growth medium per cm2 of vessel surface and gently pipet up and down to generate a single cell suspension.
- Count cells. Transfer the cell suspension into an appropriate centrifuge tube and rinse the vessel surface again with 100 μL Endothelial Cell Growth Medium per cm2 of vessel surface to collect remaining cells. Determine cell number according to your standard procedure.
- Prepare cells for tube formation assay. Prepare 15 mL centrifuge tubes (one tube per test condition) and transfer 1X105 – 1.5X105 endothelial cells in each tube. Centrifuge the tubes at 300 x g for 3 min and resuspend each pellet in 1 mL of the corresponding test or control medium.
- Prepare cells for Tube Formation Assay. Add 100 μL (= 1X104 – 1.5X104 cells) of each single cell suspension per well on top of the gelled BME. Be careful not to touch the surface of the gel.
- Incubate at 37 °C. Incubate the 96-well plate in a humidified incubator (37 °C, 5% CO2) for 4 to 24 hours. Cells can be monitored at desired time points using an inverted microscope.
Image Analysis of Tube Formation
(A) Light Microscopy
The tubular network in the wells can be imaged without fixation or labelling using an inverted microscope ( Fig. 2A and C).
(B) Calcein AM Labeling
Prepare 6 μM of Calcein AM solution (17783) in recommended basal medium. Add 50 μL of the solution per well without aspirating the medium. Incubate the plate in a humidified incubator (37 °C, 5% CO2) for 30 min. Calcein AM-labelled cells can be observed immediately using an inverted fluorescence microscope with 485 nm excitation or 520 nm emission filter.
(C) Fixation and Crystal Violet Staining
Prepare 0.1% Crystal Violet staining solution (V5265) in distilled water (w/v). Carefully remove the medium from the wells and wash the cells with 100 μL PBS per well. Remove the water and add 100 μL of Crystal Violet solution per well. Incubate the plate for 15–30 min at room temperature. Wash the cells twice using sterile water and image cells using an inverted microscope (Fig. 2D).
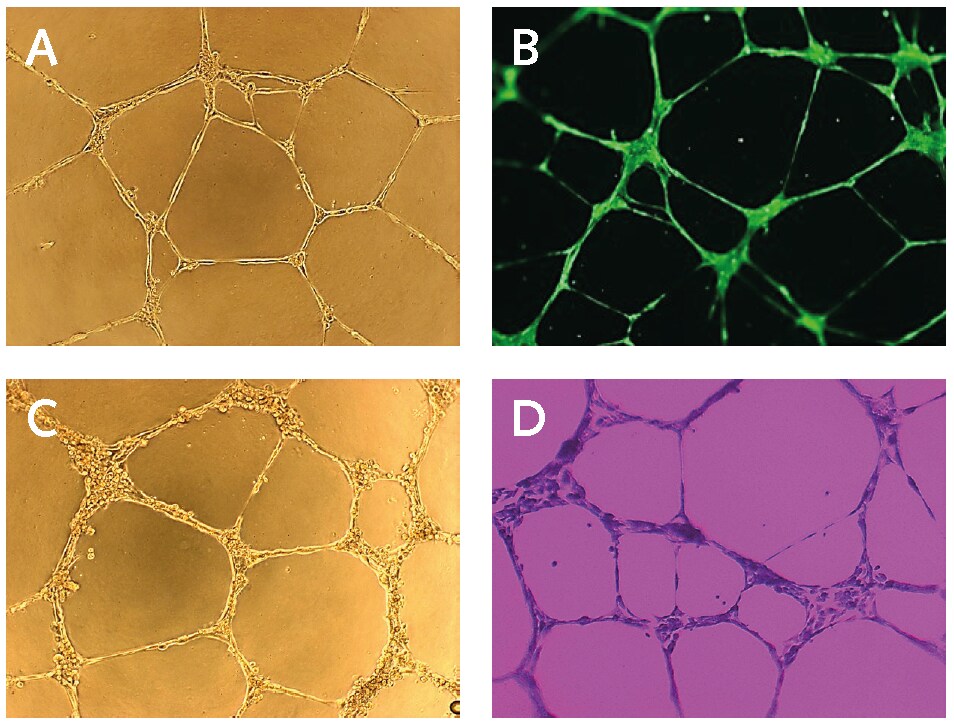
Figure 2.In vitro endothelial cell tube formation assay. (A) Light microscopy image of PromoCell HUVECs cultured on Basement Membrane Extract for 17 hours. (B) Calcein staining of PromoCell HUVECs cultured on Basement Membrane Extract for 17 hours using PromoKine’s Angiogenesis Assay Kit. (C) Light microscopy image of PromoCell HCAECs cultured on Basement Membrane Extract and stimulated with 50 ng/ml FGF for 16 hours. (D) Crystal Violet staining of PromoCell HCAECs cultured on BME for 16 hours.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?