Photocrosslinkable Hydrogels for 3D Cell Culture and Tissue Engineering
Three-dimensional (3D) cell culture systems have become increasingly valuable for biomedical research, drug discovery, and tissue engineering (TE) applications including bioprinting. Advancements in 3D cell culture that support desirable frameworks for cellular proliferation and survival can help improve recapitulation of complex in vivo environments. Hydrogels are 3D network structures held together by crosslinks, and exhibit high hydrophilicity and inherently biocompatible properties that are ideal for 3D cell culture and TE applications such as 3D bioprinting.
Natural hydrogels derived from collagen, gelatin, and hyaluronic acid have high biocompatibility, which is an intrinsic advantage for supporting cell viability, proliferation, differentiation, and locomotion. These unique compositional and structural similarities to the native extracellular matrix (ECM) can provide an effective platform for cell function, adhesion, and transplantation. Compatibility and versatility of 3D hydrogel scaffolds for cell culture and TE applications can be improved through stable polymeric networks with enhanced hydrogel crosslinking. Photoinitiated crosslinks can be formed in situ to control hydrogel stiffness and gelation rate and fabricated with varying degrees of crosslinking content under ultraviolet (UV) or visible light. Minimally invasive photocrosslinkable hydrogels are ideal for 3D cell culture and TE applications, providing exceptional temporal and spatial control.
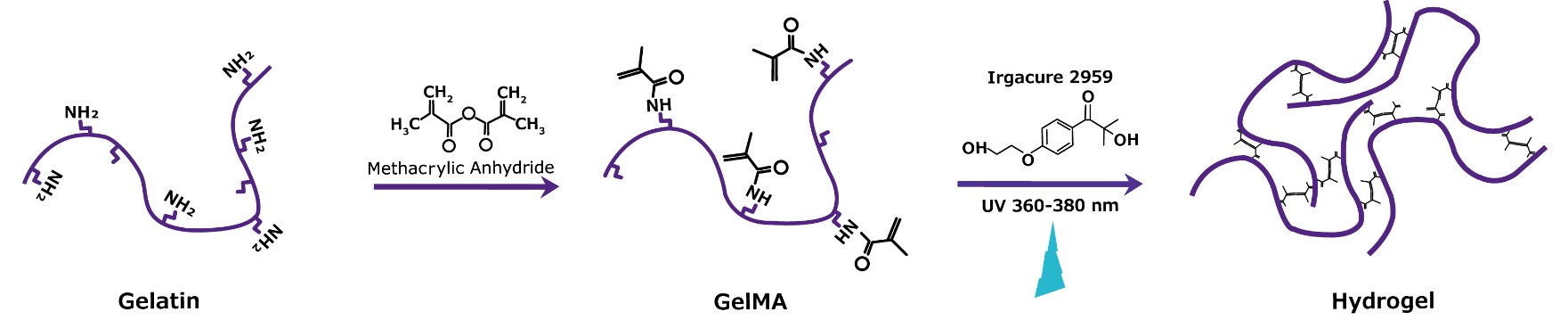
Figure 1. Fabrication of photocrosslinked GelMA hydrogel.Gelatin reacted with methacrylic anhydride (MA) to produce methacrylated gelatin (GelMA) is exposed to UV or visible light in the presence of photonitiator Irgacure® 2959 to form a hydrogel structure for 3D cell culture and bioprinting applications.
Types of Photoinitiators
Irgacure® 2959 (I2959): Highly efficient UV light-sensitive photoinitiator, with moderate water solubility, suitable for use in aqueous and photocuring systems, including hydrogels and bioinks. Irgacure® is most efficiently activated using UV light (365 nm). With well-established cytocompatibility at low concentrations, Irgacure® 2959 is non-yellowing, with low volatility and cytology, and minimal immunogenicity.
LAP (Lithium phenyl-2,4,6-trimethylbenzoylphosphinate): Water soluble and visible light-sensitive, with high efficiency and cytocompatibility. Suitable for use in hydrogel or bioink polymerization, LAP requires blue light (405 nm) for photocrosslinking. LAP supports increased cell viability and polymerization rates.
Ruthenium: Water soluble and visible light-sensitive, with covalent crosslinking at low doses of visible light (400-450 nm). Unique speed and efficiency to visible light exposure is ideal for tissue engineering, 3D cell culture, and bioprinting applications.
Photocrosslinked hydrogel kits contain high quality modified natural hydrogels including methacrylated collagen type-I (PhotoCol®), gelatin (PhotoGel®) or hyaluronic acid (PhotoHA®) along with photoinitiators (Irgacure® 2959, LAP, or Ruthenium) and optimized buffers and solutions to fit diverse 3D cell culture or bioprinting applications.

Figure 2. Bioprinting with photocrosslinked hydrogels. Photocrosslinked natural hydrogels derived from collagen, gelatin, and hyaluronic acid are used as bioinks for 3D bioprinting applications. These photocrosslinkable hydrogels facilitate enhanced biocompatibility, degradability, and bioprinting processability.

Figure 3. Physical properties of photocrosslinked hydrogels.Crosslinking PhotoHA® exposed to UV light (320-390nm) and 0.05% Irgacure® 2959 (top), compressive modulus of 1% and 3% PhotoHA® hydrogels generated stress-strain curves between 10% and 20% (bottom left), swelling characteristics of 1% and 3% PhotoHA® hydrogels imaged before and after incubation in phosphate buffered saline at 25 °C for 24 hours (bottom right).

Figure 4. Bioprinting of human mesenchymal stem cells using PhotoCol®.PhotoCol® (collagen methacrylate) can be used as a rapidly self-assembling type I collagen to form cross-linked hydrogels for tissue engineering. Collagen methacrylate is useful for forming scaffolds with varying degrees of stiffness, by altering collagen concentration or UV-light exposure. Human adipose derived mesenchymal stem cells (SCC038) (10X106 cells/mL) are pictured 1 day post bioprinting using PhotoCol® as the bioink material. Cell health was assayed using a live/dead assay (green= viable, red=dead). The majority (>90%) of the stem cells were viable after bioprinting using PhotoCol®.

Figure 5. Cells cultured in PhotoGe® hydrogels crosslinked with ruthenium.Human mesenchymal stromal cells (SCC034) (left), Human MSCs and human vein endothelial cell (HUVEC) coculture(center) and human dermal fibroblasts (right) were encapsulated in PhotoGel® hydrogels and crosslinked with ruthenium and visible light (400-450 nm).

Figure 6. 3D culture of human mesenchymal stem cells using PhotoHA®Human bone marrow mesenchymal stem cells (SCC034) (2.0 x 107 cells/mL) were encapsulated in 50 μL 1% PhotoHA® hydrogels (~4.7mm x 2mm). PhotoHA® hydrogels were crosslinked with 0.05% Irgacure® 2959 photoinitiator and exposed to 2 mW/cm2 UV light (320-390 nm) for 10 minutes. After 24 hours, encapsulated cells were stained with live/dead assay (green= viable, red=dead) and subsequently imaged on a Leica SP5 confocal microscope.

Figure 7. 3D culture of human neural stem cells using PhotoHA®Encapsulated GFP-labeled neural stem cells in 1% PhotoHA® hydrogels after 7 days in culture. HA was photoinitiated with 0.25% LAP and polymerized by exposure to UV light (365 nm) for 1 minute, to form hydrogels ~100-200 μM thick. Images at 10X magnification.
References
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?