Lentiviral Production Using X-tremeGENE HP Transfection Reagent
Lentiviruses represent a powerful tool in research applications to transduce a wide range of cell types. During virus production, low titers and toxicity can be challenging. This easy-to-use protocol uses X-tremeGENE HP Transfection Reagent for fast and robust lentiviral preparation, with high virus titers being suitable to successfully transduct target cells.
Materials
- Packaging Cell Line
TLA-HEK293T (Open Biosystems) This cell line grows adherently and is amenable to serumfree media formulations. When using X-tremeGene HP reagent, adaption to serum-free conditions is not required since this reagent is not impaired by the presence of serum. The cells should be cultured in Opti-MEMR with GlutaMAX (Invitrogen) and 10% fetal bovine serum (FBS). Maintain culture vessels at 37 °C at 10% CO2 in 95% RH. - Expression Vector
pGIPZ (Open Biosystems), or other vectors compatible with second/third generation lentivirus packaging systems encoding eGFP or the appropriate transgene. - Packaging and Envelope Plasmids
psPAX2 and pMD2.G plasmids allow expression of proteins required to produce functional virus particles, resistant to recombination.
Methods
A. Lentivirus Supernatant Production
Day 1
- Coat appropriate wells of standard 6-well cell culture plate with 1 mL of a sterile aqueous poly-lysine solution at a concentration of 0.01 mg/mL. Incubate for at least 1 hour at room temperature (+15 to +25 °C).
- Wash each well twice with 2 mL of sterile water.
- Remove water and air dry vessels under sterile conditions.
- Split TLA-HEK293T cells into coated wells at ~40-50% confluency the day before transfection, with cells growing in 2 mL growth medium (Opti-MEMR with GlutaMAX, 10% FBS).
Day 2
- Remove old media, and add 1.8 mL of fresh medium.
- Prepare transfection complex:
A) Mix library- (pGIPZ), packaging- (psPAX2), and envelope vector (pMD2.G), dissolved in sterile water or TE buffer (pH 7.6) to 2 μg in a 1:2:2 molar stoichiometry (0.53 μg; 0.95 μg,0 .52 μg).
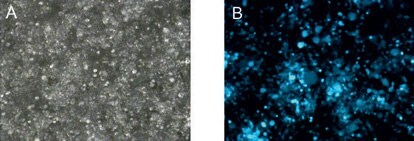
Figure 1.Transfection efficiency after 48 hours. Delivery of high amounts of viral plasmids into packaging cells using X-tremeGENE HP Transfection Reagent. (A) bright field microscopy, (B) fluorescence microscopy.
Note: Plasmid preparations should be of high quality and endotoxin free.
B) Dilute DNA into 0.2 mL transfection medium (serumfree Opti-MEM® medium).
C) Add 6 μL of X-tremeGENE HP Reagent, briefly vortex and incubate 15 minutes at room temperature.
3. Carefully add the transfection mixture to the cells and agitate gently to achieve a homogeneous distribution of compounds.
Day 3
Replace medium with 2 mL of growth medium. Note: the discarded media contains small amounts of virus particles and should be handled as waste according to biosafety level 2.
Day 5
- Collect and transfer the virus-containing supernatant to sterile 1.5 mL vial.
- Spin at 2000 × g for 10 minutes at -2 to -8 °C and transfer supernatant to new vial.
B. Determination of Virus Titers by Limited Dilution
Day 1
Seed 1 x 104 cells (here also TLA-HEK293T) per well of a poly-lysine coated 96-well plate in a volume of 200 μL of growth medium.
Day 2
- Add 40 μL of the virus-containing supernatant to 160 μL of growth medium, supplemented with 8 μg/mL Polybrene.
- Dilute 10 μL of the resulting virus solution with an additional 90 μL Polybrene-supplemented growth medium. Repeat dilution procedure at least 5 times.
- After removal of medium, add 100 μL of the dilution to the cells (cells grown to ~60% confluency).
- After 1 hour, add additional 100 μL of growth medium per well.
- Analyze cells 48 hours post transduction. Count GFP-positive cells and multiply by the dilution factor.
Results
To determine whether this protocol produced sufficiently pure and concentrated virus suitable for cell culture applications, cells were transfected according to the procedure described above. Microscopy analysis clearly revealed high levels of GFP expression (<90%) without toxicity (Figure 1).
Furthermore, the microscopy analyses following transduction using the produced virus-containing supernatant revealed yields of GFP-positive cells at 1.2 x 106 pfu/mL. Thus, the protocol established here allows the production of lentiviral viruses in suitable amounts. To avoid multiple insertions of virus DNA into the host genome, it is recommended to infect the cells at a multiplicity of infection (MOI) below 0.1. This protocol provides virus amounts derived from one well of a standard 6-well plate for the transduction of at least 107 cells.
For life science research only. Not for use in diagnostic procedures.
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?