About This Item
Código UNSPSC:
12352108
NACRES:
NA.32
Productos recomendados
concentración
≥10.0 mg/mL (UV)
técnicas
electrophoresis: suitable
fluorescencia
λex 609 nm; λem 643 nm in 0.1 M phosphate pH 7.2
idoneidad
in accordance for gel electrophoresis
temp. de almacenamiento
2-8°C
Descripción general
The product is suspended in 150 mM sodium phosphate, 60% ammonium sulfate, 1 mM EDTA, 1 mM sodium azide, pH 7.0 and must be dialyzed against conjugation buffer or PBS before conjugation.
Aplicación
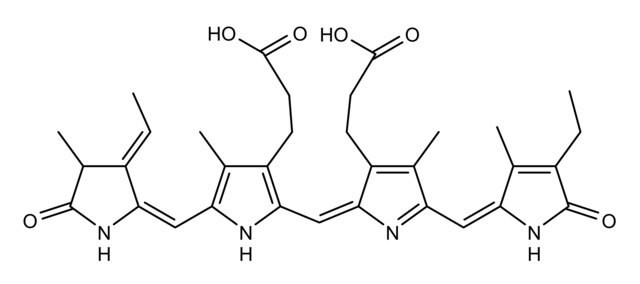
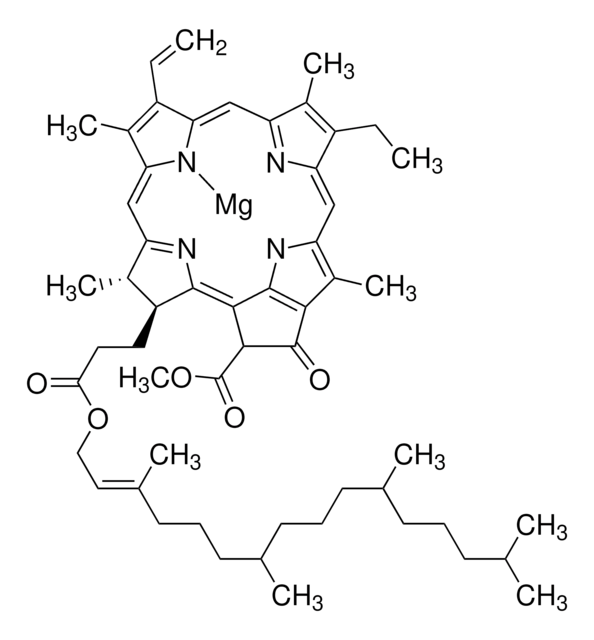
C-Phycocyanin (CPC), a pigment-protein complex from the light-harvesting phycobiliprotein family, may be used in immunoassay kit development and to study its properties as a light harvesting protein.
Envase
Bottomless glass bottle. Contents are inside inserted fused cone.
Nota de análisis
A620/A280 >3.5, A651/A620 <0.3
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Faceshields, Gloves, Goggles
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
H Scheer et al.
Molecular microbiology, 68(2), 263-276 (2008-02-21)
Biliproteins are a widespread group of brilliantly coloured photoreceptors characterized by linear tetrapyrrolic chromophores, bilins, which are covalently bound to the apoproteins via relatively stable thioether bonds. Covalent binding stabilizes the chromoproteins and is mandatory for phycobilisome assembly; and, it
Jordan M Womick et al.
The journal of physical chemistry. B, 113(48), 15771-15782 (2009-11-12)
The electronic structure and photoinduced relaxation dynamics of the cyanobacterial light harvesting protein, C-Phycocyanin (CPC), are examined using transient grating and two-dimensional (2D) photon echo spectroscopies possessing sub-20 fs time resolution. In combination with linear absorption and fluorescence measurements, these
Michaela Kupka et al.
Biochimica et biophysica acta, 1777(1), 94-103 (2007-11-27)
Optical spectroscopic properties of the covalently linked chromophores of biliproteins are profoundly influenced by the state of the protein. This has been used to monitor the urea-induced denaturation of C-phycocyanin (CPC) from Mastigocladus laminosus and its subunits. Under equilibrium conditions
Lijin Tian et al.
The journal of physical chemistry. B, 117(38), 11000-11006 (2012-12-18)
Cyanobacteria are oxygen-evolving photosynthetic organisms that harvest sunlight and convert excitation energy into chemical energy. Most of the light is absorbed by large light harvesting complexes called phycobilisomes (PBs). In high-light conditions, cyanobacteria switch on a photoprotective mechanism called non-photochemical
Rasiah Pratheepa Kumari et al.
Biological trace element research, 151(1), 59-67 (2012-10-23)
The present investigation is aimed to evaluate the anticataractogenic potential of C-phycocyanin (C-PC), extracted and purified from Spirulina platensis. Enucleated rat lenses were maintained in vitro in Dulbecco's modified Eagle medium (DMEM). Group I contained DMEM, Group II and Group
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico