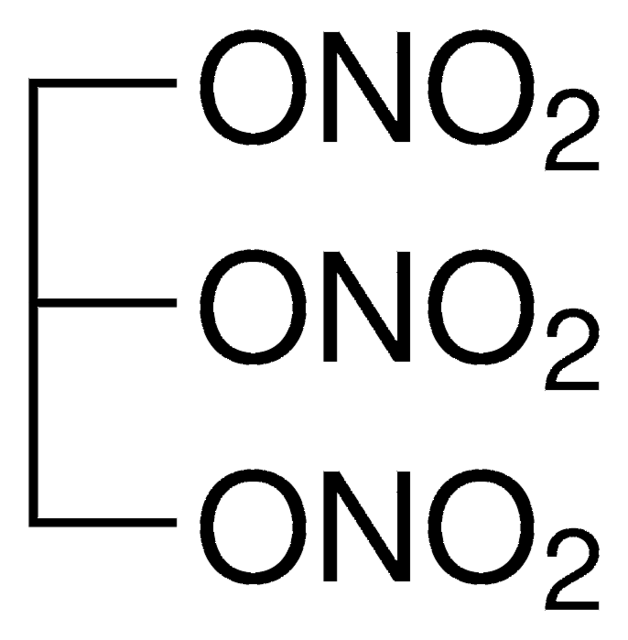
D-011
1,3-Dinitroglycerin solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
Nivel de calidad
formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 1 mL
fabricante / nombre comercial
Cerilliant®
concentración
1.0 mg/mL in acetonitrile
técnicas
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicaciones
pharmaceutical (small molecule)
formato
single component solution
temp. de almacenamiento
−20°C
cadena SMILES
OC(CO[N+]([O-])=O)CO[N+]([O-])=O
InChI
1S/C3H6N2O7/c6-3(1-11-4(7)8)2-12-5(9)10/h3,6H,1-2H2
Clave InChI
ASIGVDLTBLZXNC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Population pharmacokinetics of nitroglycerin and of its two metabolites after a single 24-hour application of a nitroglycerin transdermal matrix delivery system: This study presents a comprehensive analysis on the population pharmacokinetics of nitroglycerin, focusing on its behavior and the behavior of its metabolites following the use of a transdermal delivery system. The findings contribute to understanding the systemic availability and action of nitroglycerin from such systems, which could be relevant for 1,3-Dinitroglycerin given its structural and functional similarities (Auclair et al., 1998).
- Novel pharmacokinetic modelling of transdermal nitroglycerin: This research explores advanced pharmacokinetic models that describe the absorption and action of nitroglycerin when administered through transdermal patches. Such models are crucial for developing effective transdermal delivery systems for similar compounds like 1,3-Dinitroglycerin (Auclair et al., 1998).
- Bioequivalence Comparison of Two Drug-in-Adhesive Transdermal Nitroglycerin Patches: This study compares the bioequivalence of two transdermal patches delivering nitroglycerin, highlighting the critical factors in the design and performance of transdermal systems that could similarly apply to 1,3-Dinitroglycerin (Harrison et al., 1996).
- Percutaneous absorption of 1,3-dinitroglycerin and a trial of pharmacokinetic analysis: Directly relevant to 1,3-Dinitroglycerin, this study examines its percutaneous absorption characteristics, offering insights into its potential applications in transdermal delivery systems (Ogiso et al., 1990).
- Analysis of solutions containing glutathione and inorganic nitrite: application to nitroglycerin metabolism studies: While primarily focused on nitroglycerin, this analysis could provide secondary insights into the metabolic pathways and interactions of 1,3-Dinitroglycerin with biomolecules like glutathione, which is crucial for understanding its pharmacological effects (Curry et al., 1987).
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
35.6 °F - closed cup
Punto de inflamabilidad (°C)
2 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico