D34108
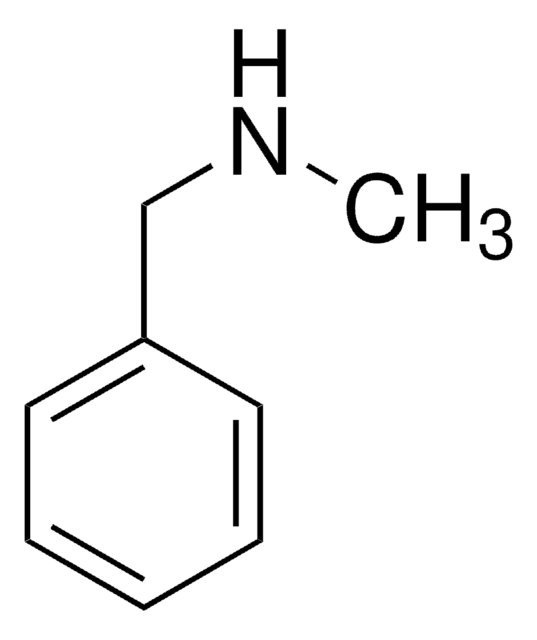
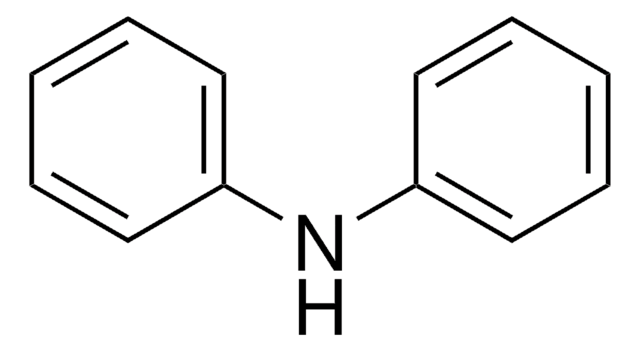
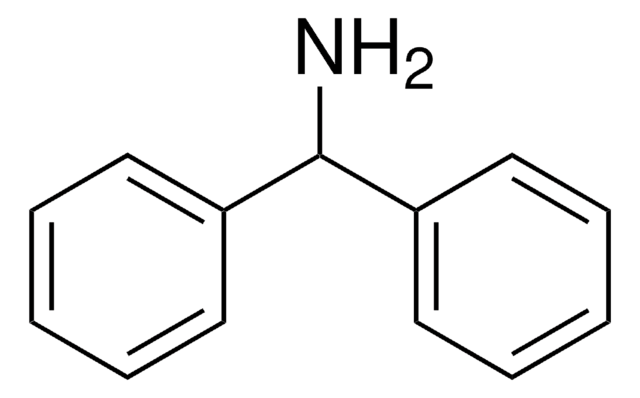
Dibenzylamine
97%
Sinónimos:
(N-Benzylaminomethyl)benzene, Bibenzylamine, DBA, Dibenzylamine (8CI), N,N-Dibenzylamine, N-(Phenylmethyl)benzenemethanamine, N-Benzyl-1-phenylmethanamine, N-Benzylbenzylamine
About This Item
Productos recomendados
Análisis
97%
formulario
liquid
índice de refracción
n20/D 1.574 (lit.)
bp
300 °C (lit.)
mp
−26 °C (lit.)
densidad
1.026 g/mL at 25 °C (lit.)
cadena SMILES
C(NCc1ccccc1)c2ccccc2
InChI
1S/C14H15N/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2
Clave InChI
BWLUMTFWVZZZND-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Environmental monitoring in shale gas wastewater: Dibenzylamine was identified among the hazardous substances in shale gas wastewater, with research characterizing its concentration and distribution in the Upper Yangtze River, contributing to improved environmental management practices (Tang et al., 2024).
- Advancements in organic synthesis: Dibenzylamine was used in a novel synthetic strategy for (L)-Monomethyl Tyrosine via bulky ′forced-traceless′ regioselective Pd-catalyzed C(sp(2))-H activation, showcasing its utility in pharmaceutical compound development (Illuminati et al., 2023).
- Application in crystallography: The crystal structure of di-benzyl-ammonium was elucidated, providing insights into molecular interactions and potential applications in material science and drug design (Traoré et al., 2023).
- Utilization in green chemistry: Dibenzylamine facilitated a green approach towards Triazole forming reactions, aiming to develop anticancer drugs by minimizing environmental impact and enhancing reaction efficiency (Rastogi et al., 2023).
Palabra de señalización
Danger
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
289.4 °F - closed cup
Punto de inflamabilidad (°C)
143 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico